-
摘要: 研究了丙烯在金属铁作用下还原NO的特性。采用陶瓷管流动反应器在300-1100℃研究了不同条件下的NO还原效率,考察了SO2的影响,采用XRD、SEM和EDS分析了反应后金属铁表面的组分和微观结构特征。结果表明,丙烯在金属铁作用下具有良好的NO还原效果。在N2气氛,温度超过800℃后,金属铁作用下丙烯还原NO的效率达到了95%以上。在模拟烟气、富燃料条件下,温度高于900℃时,丙烯与金属铁还原NO的效率超过了90%。SO2对丙烯在金属铁作用下还原NO的效率影响很小。机理分析表明,当丙烯与金属铁共同还原NO时,一方面,NO被金属铁直接还原,同时丙烯还原氧化铁为金属铁;另一方面,丙烯通过再燃机理还原NO,同时再燃中间产物被氧化铁氧化为N2。Abstract: NO reduction by propene with iron was experimentally studied. NO reduction efficiency at different conditions was tested in a flow-type ceramic tube reactor at 300-1100℃. The effect of SO2 was also investigated. XRD, SEM and EDS techniques were used to analyze the composition and surface microstructure of the iron sample after reaction. Results showed that propene was effective to reduce NO with iron. More than 95% of NO was reduced above 800℃ in N2 atmosphere and more than 90% of NO was reduced above 900℃ at fuel-rich conditions in simulated flue gas atmosphere by propene with iron respectively. SO2 had minor effect on NO reduction. The analysis on reaction mechanism showed that when propene was used to reduce NO with iron, on one hand, NO was directly reduced by iron while propene reduced the iron oxides to iron; on the other hand, propene reduced NO via reburning reaction while the re-burning intermediates were oxidized by iron.
-
Key words:
- combustion emission control /
- flue gas deNOx /
- C3H6 /
- iron
-
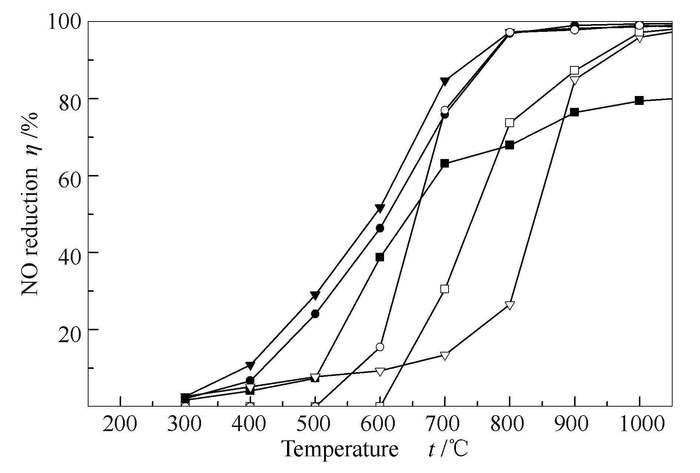
图 6 模拟烟气条件下C3H6-Fe还原NO的效率
Figure 6 NO reduction by propene over metallic iron in simulated flue gas atmosphere
(flow rate 2 L/min, φO2=2.0%,φCO2=17.0%, φNO=0.05%, in N2 base)
■: SR1=SR2=0.7; □: SR1=0.7, SR2=1.2;●: SR1=SR2=0.8; ○: SR1=0.8, SR2=1.2;
▲: SR1=SR2=0.9; △: SR1=0.9, SR2=1.2;
▼: SR1=SR2=1.0; ∇: SR1=1.0, SR2=1.2;
◀: SR1=SR2=1.1; ◁: SR1=1.1, SR2=1.2;▶: SR1=SR2=1.2图 7 模拟烟气条件下有、无铁时C3H6还原NO的效率对比
Figure 7 Comparison of NO reduction by propene with and without iron in simulated flue gas atmosphere
(flow rate 2 L/min, φO2=2.0%, φCO2=17.0%, φNO=0.05%, in N2 base)
■: with iron, SR1=0.7, SR2=1.2;
□: without iron, SR1=0.7, SR2=1.2;
●: with iron, SR1=0.9, SR2=1.2;
○: without iron, SR1=0.9, SR2=1.2;
▲: with iron, SR1=1.0, SR2=1.2;
△: without iron, SR1=1.0, SR2=1.2;
▼: with iron, SR1=1.2, SR2=1.2;
∇: without iron, SR1=1.2, SR2=1.2图 8 模拟烟气条件下金属铁作用下C3H6与C3H8还原NO的效率对比
Figure 8 Comparison of NO reduction by propene and propane with iron in simulated flue gas atmosphere
(flow rate 2 L/min, φO2=2.0%, φCO2=17.0%, φNO=0.05%, in N2 base)
■: C3H6, SR1=0.7, SR2=1.2;
□: C3H8, SR1=0.7, SR2=1.2;
●: C3H6, SR1=0.9, SR2=1.2;
○: C3H8, SR1=0.9, SR2=1.2;
▲: C3H6, SR1=1.0, SR2=1.2;
△: C3H8, SR1=1.0, SR2=1.2;
▼: C3H6, SR1=1.2, SR2=1.2;
∇: C3H8, SR1=1.2, SR2=1.2图 9 模拟烟气条件下SO2对金属铁作用下C3H6还原NO效率的影响 (SR1=0.9)
Figure 9 Effect of SO2 on the reduction of NO by propene over iron in simulated flue gas atmosphere
(flow rate 2 L/min, φO2=2.0%, φCO2=17.0%, φNO=0.05%, φSO2=0-0.04%, in N2 base)
■: φSO2=0, SR2=1.2;
□: φSO2=0, SR2=0.9;
●: φSO2=0.01%, SR2=1.2;
○: φSO2=0.01%, SR2=0.9;
▲: φSO2=0.02%, SR2=1.2;
△: φSO2=0.02%, SR2=0.9;
▼: φSO2=0.04%, SR2=1.2;
∇: φSO2=0.04%, SR2=0.9表 1 N2氛围中φC3H6为0.2%作用下铁还原NO后铁样品EDS表征
Table 1 EDS results over iron surface after reducing NO with φC3H6=0.2% in N2 atmosphere
Image 1 Image 2 element w/% atomic percent watom/% element w/% atomic percent watom/% C 2.60 9.08 C 3.86 12.38 O 9.65 25.22 O 12.36 29.78 Fe 87.75 65.71 Fe 83.78 57.84 Total 100.00 100.00 Total 100.00 100.00 -
[1] LIU J, LI X, ZHAO Q, ZHANG D, NDOKOYE P.The selective catalytic reduction of NO with propene over Cu-supported Ti-Ce mixed oxide catalysts:Promotional effect of ceria[J].J Mol Catal A:Chem, 2013, 378:115-123. doi: 10.1016/j.molcata.2013.06.005 [2] LIU X, JIANG Z, CHEN M, SHI J, SHANGGUAN W, TERAOKA Y.Characterization and performance of Pt/SBA-15 for low-temperature SCR of NO by C3H6[J].J Environ Sci, 2013, 25(5):1023-1033. doi: 10.1016/S1001-0742(12)60107-7 [3] HOUEL V, JAMES D, MILLINGTON P, POLLINGTON S, POULSTON S, RAJARAM R, TORBATI R.A comparison of the activity and deactivation of Ag/Al2O3 and Cu/ZSM-5 for HC-SCR under simulated diesel exhaust emission conditions[J].J Catal, 2005, 230(1):150-157. doi: 10.1016/j.jcat.2004.12.003 [4] KOMVOKIS V G, ILIOPOULOU E F, VASALOS I A, TRIANTAFYLLIDIS K S, MARSHALL C L.Development of optimized Cu-ZSM-5 deNOx catalytic materials both for HC-SCR applications and as FCC catalytic additives[J].Appl Catal A:Gen, 2007, 325(2):345-352. doi: 10.1016/j.apcata.2007.02.035 [5] BURCH R, WATLING T C.The effect of promoters on Pt/Al2O3 catalysts for the reduction of NO by C3H6 under lean-burn conditions[J].Appl Catal B:Environ, 1997, 11(2):207-216. doi: 10.1016/S0926-3373(96)00043-4 [6] CHANG F Y, WEY M Y, CHEN J C.Effects of sodium modification, different reductants and SO2 on NO reduction by Rh/Al2O3 catalysts at excess O2 conditions[J].J Hazard Mater, 2008, 156(1/3):348-355. [7] ZHANG R, VILLANUEVA A, ALAMDARI H, KALIAGUINE S.Cu- and Pd- substituted nanoscale Fe-based perovskites for selective catalytic reduction of NO by propene[J].J Catal, 2006, 237(2):368-380. doi: 10.1016/j.jcat.2005.11.019 [8] 王新葵, 张万生, 王爱琴, 王晓东, 杨学锋.Au/Fe2O3/Al2O3催化剂上丙烯选择催化还原NO[J].催化学报, 2008, 29(6):503-505. doi: 10.1016/S1872-2067(08)60046-7WANG Xin-kui, ZHANG Wan-sheng, WANG Ai-qin, WANG Xiao-dong, YANG Xue-feng, ZHANG Tao.Selective catalytic reduction of NO with propene over Au/Fe2O3/Al2O3 catalysts[J].Chin J Catal, 2008, 29(6):503-505. doi: 10.1016/S1872-2067(08)60046-7 [9] 叶青, 闫立娜, 霍飞飞, 王海平, 程水源, 康天放.Fe柱撑海泡石负载Cu催化剂:结构特点及其C3H6选择性催化还原NO催化性质[J].无机化学学报, 2012, 28(1):103-112.YE Qing, YAN Li-na, HUO Fei-fei, WANG Hai-ping, CHENG Shui-yuan, KANG Tian-fang.Cu-supported on Fe-pillared sepiolite:Characterization and selective catalytic reduction (SCR) of NO by propene[J].Chin J Inorg Chem, 2012, 28(1):103-112. [10] 郭锡坤, 刘庆红, 林绮纯.镧改性铜基铝铈交联蒙脱土的制备及其对丙烯选择性还原NO的催化性能[J].催化学报, 2004, 25(12):989-994. http://www.cnki.com.cn/Article/CJFDTOTAL-CHUA200412012.htmGUO Xi-kun, LIU Qing-hong, LIN Qi-chun.Preparation of La-modified Cu-based Al-Ce pillared montmorillonite and its catalytic properties for selective reduction of NO by propylene[J].Chin J Catal, 2004, 25(12):989-994. http://www.cnki.com.cn/Article/CJFDTOTAL-CHUA200412012.htm [11] 苏亚欣, 任立铭, 苏阿龙, 邓文义.甲烷在金属铁及氧化铁表面还原NO的研究[J].燃料化学学报, 2013, 41(11):1393-1400. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18303.shtmlSU Ya-xin, REN Li-ming, SU A-long, DENG Wen-yi.Experimental study on NO reduction by methane over iron and its oxides[J].J Fuel Chem Technol, 2013, 41(11):1393-1400. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18303.shtml [12] 苏亚欣, 陆哲惺, 周皞, 窦逸峰, 邓文义.丙烷在金属铁表面还原NO的实验研究[J].燃料化学学报, 2014, 42(12):1470-1477. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18540.shtmlSU Ya-xin, LU Zhe-xing, ZHOU Hao, DOU Yi-feng, DENG Wen-yi.Experimental study on NO reduction by propane over iron[J].J Fuel Chem Technol, 2014, 42(12):1470-1477. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18540.shtml [13] 窦逸峰, 苏亚欣, 陆哲惺, 周皞, 邓文义.乙烷在金属铁表面还原NO的实验研究[J].燃料化学学报, 2015, 43(10):1273-1280. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18719.shtmlDOU Yi-feng, SU Ya-xin, LU Zhe-xing, ZHOU Hao, DENG Wen-yi.Experimental study of NO reduction by ethane over iron[J].J Fuel Chem Technol, 2015, 43(10):1273-1280. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18719.shtml [14] 苏亚欣, 苏阿龙, 任立铭, 邓文义.SO2对甲烷在金属铁表面还原NO的影响[J].燃料化学学报, 2014, 42(3):377-384. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18382.shtmlSU Ya-xin, SU A-long, REN Li-ming, DENG Wen-yi.Effect of SO2 on NO reduction by methane over iron[J].J Fuel Chem Technol, 2014, 42(3):377-384. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18382.shtml [15] 周皞, 苏亚欣, 戚越舟, 陆哲惺, 邓文义.水蒸气对甲烷在金属铁表面还原NO的影响[J].燃料化学学报, 2014, 42(11):1378-1386. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18528.shtmlZHOU Hao, SU Ya-xin, QI Yue-zhou, LU Zhe-xing, DENG Wen-yi.Effect of water vapor on NO reduction by methane over iron[J].J Fuel Chem Technol, 2014, 42(11):1378-1386. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18528.shtml [16] GRADON B, LASEK J.Investigation of reduction of NO to N2 by reaction with Fe[J].Fuel, 2010, 89(11):3505-3509. doi: 10.1016/j.fuel.2010.06.020 [17] 苏亚欣, 苏阿龙, 成豪.CO对铁丝网卷还原NO的影响实验研究[J].应用基础与工程科学学报, 2013, 21(4):638-646. http://www.cnki.com.cn/Article/CJFDTOTAL-YJGX201304007.htmSU Ya-xin, SU A-long, CHENG Hao.Experimental study on effect of CO on NO reduction by iron mesh roll[J].J Basic Sci Eng, 2013, 21(4):638-646. http://www.cnki.com.cn/Article/CJFDTOTAL-YJGX201304007.htm [18] DAGAUT P, LUCHE J, CATHONNET M.Experimental and kinetic modeling of the reduction of NO by propene at 1 atm[J].Combust Flame, 2000, 121(4):651-661. doi: 10.1016/S0010-2180(00)00095-X [19] KOUOTOU P M, TIAN Z, VIEKER H, BEYER A, GOLZHAUSER A, KOHSE-HOINGHAUS K.Selective synthesis of a-Fe2O3 thin films and effect of the deposition temperature and lattice oxygen on the catalytic combustion of propene[J].J Mater Chem A, 2013, 1:10495-10504. doi: 10.1039/c3ta11354j [20] BALDI M, ESCRIBANO V S, AMORES J M G, MILELLA F, BUSCA G.Characterization of manganese and iron oxides as combustion catalysts for propane and propene[J].Appl Catal B:Environ, 1998, 17(3):L175-L182. doi: 10.1016/S0926-3373(98)00013-7 [21] VAN STEEN E, SCHNOBEL M, WALSH R, Riedel T.Time on stream behaviour in the partial oxidation of propene over iron antimony oxide[J].Appl Cata A:Gen, 1997, 165:349-356. doi: 10.1016/S0926-860X(97)00217-2 [22] JOHNSON D K, ENGELHARDT D A, HARVILLA J.Reburning technologies for the control of nitrogen oxides emissions from coal-fired boilers[R].Washington, DC:United States Dept.of Energy, 1999. [23] 苏亚欣, GATHITU B B, CHEN W-Y.Fe2O3控制再燃脱硝中间产物HCN的实验研究[J].环境科学学报, 2011, 31(6):1181-1186.SU Ya-xin, GATHITU B B, CHEN W Y.Experimental examination of HCN compound control by Fe2O3 during reburning processes[J].Acta Sci Circumstantiae, 2011, 31(6):1181-1186. [24] SU Y, GATHITU B B, CHEN W Y.Efficient and cost effective reburning using common wastes as fuel and additives[J].Fuel, 2010, 89(9):2569-2582. doi: 10.1016/j.fuel.2009.12.009 -





 下载:
下载: