Effects of tungsten, molybdenum and nickel content chang on physicochemical properties and hydrogenation activity of bulk catalysts
-
摘要: 制备不同活性金属原子比的体相催化剂,通过BET、XRD、SEM、TEM、强度测定、堆积密度测定及小型活性评价手段,考察了活性金属钨、钼、镍含量的变化对体相催化剂物化性质和活性的影响。结果表明,保持W/Mo原子比不变,随着(W+Mo)/Ni的原子比减小,孔体积、比表面积、孔径增大,超深度加氢脱硫活性增强,在精制油硫含量小于10 μg/g,反应温度降低8 ℃。在(W+Mo)/Ni的原子比不变的条件下,W/Mo的原子比在0.28-1.85,随着原子比增大,孔体积、比表面积、超深度加氢脱硫活性没有明显变化。Abstract: Bulk catalysts with different active metal atomic ratios were prepared. The effects of changing active metal tungsten, molybdenum and nickel contents on the physicochemical properties and activity of bulk catalysts were investigated using various characterization instruments, such as BET, XRD, SEM, TEM, determination of strength, packing density and laboratory scale hydrogenation apparatus. The results show that pore volume, specific surface area, pore size and the activity of ultra-deep hydrodesulfurization were all increased with the decrease of (W+Mo)/Ni ratio, while W/Mo atomic ratio remained constant. The reaction temperature reduced by 8℃ when sulfur content of refined oil is less than 10 μg/g. And the pore volume, specific surface area, pore size and ultra-deep hydrodesulfurization activity did not change obviously with increase of the W/Mo atomic ratio in the range of 0.28-1.85 while the (W+Mo)/Ni atomic ratio was kept unchanged.
-
Key words:
- bulk catalyst /
- physicochemical properties /
- metal content /
- hydrotreating activity /
- mole ratio
-
表 1 不同第ⅥB族金属与第Ⅷ族金属原子比(λ1 )体相催化剂的物化性质
Table 1 Physic-chemical properties of different (W+Mo)/Ni bulk catalysts
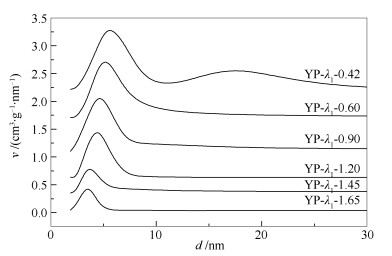
Catalyst YP-λ1-0.42 YP-λ1-0.60 YP-λ1-0.90 YP-λ1-1.20 YP-λ1-1.45 YP-λ1-1.65 YP-λ1-1.90 λ1 0.42 0.60 0.90 1.20 1.45 1.65 1.90 NiO/% 38 30 24 18 15 12.3 9 vP/(cm3·g-1) 0.385 0.350 0.299 0.271 0.250 0.235 0.186 ABET/(m2·g-1) 223 208 195 184 168 151 125 dP/nm 7.0 6.5 6.1 5.6 4.8 4.3 3.5 Stack density ρ/(g·cm-3) 0.80 0.86 0.93 1.05 1.20 1.26 1.38 Crush strength/(N·mm-1) 7.0 9.7 14.5 16.2 17.2 19.5 20.8 表 2 不同λ1催化剂WS2/MoS2片层平均长度和平均堆叠层数
Table 2 Average slab layers and slab length of different (W+Mo)/Ni sulfurized bulk catalysts
Catalyst Slab layers N Slab length L/nm YP-λ1-0.60 5.71 7.99 YP-λ1-0.90 5.62 7.88 YP-λ1-1.20 4.19 7.90 YP-λ1-1.45 3.59 7.91 表 3 不同λ1体相催化剂镇海常三线柴油的对比
Table 3 Catalyst activity evaluation of different (W+Mo)/Ni bulk catalysts
Catalyst YP-λ1-0.90 YP-λ1-1.20 YP-λ1-1.45 Reaction temperature t/℃ base base +5 base +8 Properties of feedstock and refined oil material refining oil refining oil refining oil Density(20 ℃) ρ/(g·cm-3) 0.859 6 0.830 3 0.829 6 0.830 0 Boiling range (ASTM-D86) t/℃(IBP-EBP) 202-373 181-373 156-373 166-373 w(S)/(μg·g-1) 14 424 8.5 9.2 9.0 w(N)/(μg·g-1) 389 1.0 1.0 1.0 w(aromatic hydrocarbons)/% 37 11.9 11.6 11.7 w(polycyclic aromatic hydrocarbons)/% 20.5 2.0 1.8 1.8 Cetane number 50.8 55.9 55.2 55.4 表 4 不同λ2体相催化剂的物化性质
Table 4 Physic-chemical properties of different W/Mo bulk catalyst
Catalyst YP-λ2-0.28 YP-λ2-0.60 YP-λ2-1.35 YP-λ2-1.85 YP-λ2-2.05 λ2 0.28 0.60 1.35 1.85 2.05 vP/(cm3·g-1) 0.284 0.276 0.278 0.285 0.346 ABET/(m2·g-1) 182 175 181 185 218 dP/nm 5.7 5.6 5.7 5.8 7.5 Stack density ρ/(g·cm-3) 1.03 1.04 1.05 1.03 0.79 Crush strength/(N·mm-1) 14.3 15.1 14.8 14.2 7.2 表 5 不同λ2硫化态催化剂WS2/MoS2片层平均长度和平均堆叠层数
Table 5 Average slab layer and slab length of different W/Mo sulfurized bulk catalysts
Catalyst Slab layers N Slab length L/nm YP-λ2-0.28 5.58 7.91 YP-λ2-0.60 5.62 7.95 YP-λ2-1.35 5.64 7.99 YP-λ2-1.85 5.61 7.93 表 6 不同λ2体相催化剂的加氢活性评价
Table 6 Catalyst activity evaluation of different W/Mo bulk catalysts
Catalyst YP-λ2-0.28 YP-λ2-0.60 YP-λ2-1.35 YP-λ2-1.85 Reaction temperature t/℃ base base base base Properties of feedstock and refined oil material refining oil refining oil refining oil refining oil Density(20 ℃) ρ/(g·cm-3) 0.866 6 0.838 1 0.838 9 0.838 7 0.838 9 Boiling range (ASTM-D86) t/℃(IBP-EBP) 194-365 176-360 176-359 177-359 176-360 w(S)/(μg·g-1) 13 000 7.7 7.5 7.8 8.0 w(N)/(μg·g-1) 631 1.0 1.0 1.0 1.0 w(aromatic hydrocarbons)/% 25.2 16.6 16.5 16.7 16.4 w(polycyclic aromatic hydrocarbons)/% 13.6 3.1 3.1 3.0 3.0 Cetane number 44.5 51.4 51.2 51.3 51.2 -
[1] HUIRACHE-ACUNA R, ALONSO-NUNEZ G, PARAGUAY-DELGELGADO ARAGUAY F, LARA-ROMERO J, BERHAULT G, RIUVERA-MUNOZ E M. Unsupported trimetallic CoMoW sulfide HDS catalysts prepared by in situ decomposition of sulfur-containing precursors[J]. Catal Today, 2015, 250(7):28-37. https://www.sciencedirect.com/science/article/pii/S0920586114004969 [2] NAVA H, PEDRAZA F, ALONSO G. Ni-Mo-W sulfide catalysts prepared by in situ activation of tri-metallic alkylthiomolybdotungstates[J]. Catal Lett, 2005(1/2), 99:65-71. doi: 10.1007/s10562-004-0777-1 [3] 李灿, 蒋宗轩, 王璐. 用于柴油加氢脱硫的本体多金属及其制法和应用: 中国, 101153228[P]. 2008-04-02.LI Chan, JIANG Zong-xuan, WANG Lu. The preparation and application of bulk metal for diesel hydrodesulfurization: CN, 10115322[P]. 2008-04-02. [4] EIJSBOUTS S, MAYO S W, FUJITA F. Unsupported transition metal sulfide catalysts:from fundamentals to industrial application[J]. Appl Catal A:Gen, 2007, 322(4):58-66. https://www.sciencedirect.com/science/article/pii/S0926860X07000087 [5] 王海涛, 徐学军, 刘东香, 冯小萍, 陈光, 王继峰. FTX体相柴油超深度加氢脱硫催化剂的研制[J].工业催化, 2012, 20(6):32-35. http://d.wanfangdata.com.cn/Periodical_gych201206007.aspxWANG Hai-tao, XU Xue-jun, LIU Dong-xiang, FENG Xiao-ping, CHEN Guang, WANG Ji-feng. Preparation of high active bulk catalyst for hydrorefining[J]. Ind Catal, 2012, 20(6):32-35. http://d.wanfangdata.com.cn/Periodical_gych201206007.aspx [6] WANG L, ZHANG Y N, ZHANG Y L, JIANG Z X, LI C. Ultra-deep hydrodesulfurization of diesel fuels on trimetallic NiMoW sulfide catalysts[J]. Chem Eur J, 2009, 15(16):12571-12575. http://cat.inist.fr/?aModele=afficheN&cpsidt=22185261 [7] WANG L, ZHANGY N, ZHANG Y L, LIU P, HAN H X, YANG, M, JIANG Z X, LI C. Hydrodesulfurization of 4, 6-DMDBT on a multi-metallic sulfide catalyst with layered structure[J]. Appl CatalA:Gen, 2011, 394(1/2):18-24. https://www.sciencedirect.com/science/article/pii/S0926860X10008100 [8] LICEA Y E, GRAU-CRESPOR, PALACIOL P, JR FARO A C. Unsupported trimetallic Ni(Co)-Mo-W sulphide catalysts prepared from mixed oxides:Characterisation and catalytic tests for simultaneous tetralin HDA and dibenzothiophene HDS reactions[J]. Catal Today, 2017, 292(9):84-86 [9] 周桦, 王海涛, 徐学军. FTX催化剂在柴油加氢精制装置上的工业应用[J].炼油技术与工程, 2016, 46(4):47-50. http://d.wanfangdata.com.cn/Periodical_lysj201208012.aspxZHOU Hua, WANG Hai-tao, XU Xue-jun. The commercial application FTX catalyst in diesel hydrotreating unit[J]. Petrol Refin Eng, 2016, 46(4):47-50. http://d.wanfangdata.com.cn/Periodical_lysj201208012.aspx [10] SONG C S. An overview of new approaches to deep desulfurization for ultra-clean gasoline, diesel fuel and jet fuel[J]. Catal Today, 2003, 86(1/4):211-263. https://www.sciencedirect.com/science/article/pii/S0920586103004127 [11] ZHANG B S, YI Y J, ZHANG W, LIANG C H, SU D S, Electron microscopy investigation of the microstructure of unsupported Ni-Mo-W sulfide[J]. Mater Charact, 2011, 62(7):684-690. doi: 10.1016/j.matchar.2011.04.022 [12] JENNIFER HEIN, LIVER Y GUTIERREZ, EVA SCHACHTL, XU P H, NIGEL D BROWNING, ANDREAS JENTYS, JOHANNES A LERCHER. Distribution of metal cations in Ni-Mo-W sulfide catalysts[J]. ChemCatChem, 2015, 7(22):3692-3704. doi: 10.1002/cctc.201500788 [13] 孔研, 殷长龙, 柳云琪, 赵瑞玉, 张孔远, 王嘉玮, 刘晨光.非负载型NiMoW加氢催化剂的制备、表征和性能评价[J].石油学报(石油加工), 2012, 28(5):730-738. http://www.cnki.com.cn/Article/CJFDTOTAL-SXJG201205006.htmKONG Yan, YING Chang-long, LIU Yun-qi, ZHAO Rui-yu, ZHANG Kong-yuan, WANG Jia-wei, LIU Chen-guang. Preparation, characterization and catalytic performance of unsupported NiMoW hydrotreating catalyst[J]. Acta Pet Sin (Pet Process Sect), 2012, 28(5):730-738. http://www.cnki.com.cn/Article/CJFDTOTAL-SXJG201205006.htm [14] 曾鹤, 李鹤鸣, 王晨, 施岩, 王海彦.老化成胶和磷改性对非负载型催化剂结构和性能的影响[J].石油化工, 2016, 45(2):181-187. http://www.cnki.com.cn/Article/CJFDTOTAL-GYCH201207011.htmZENG He, LI He-ming, WANG Chen, SHI Yan, WANG Hai-yan. Effects of aging time, gelling temperature and phosphorus modification on unsupported Ni-Mo catalysts for hydrodesulfurization[J]. Petrochem, Technol, 2016, 45(2):181-187. http://www.cnki.com.cn/Article/CJFDTOTAL-GYCH201207011.htm [15] 邵长丽, 殷长龙, 张俊萍, 陈世安, 刘晨光.尿素反应法制备介孔Ni.Mo复合氧化物[J].无机化学学报, 2008, 24(11):1782-1788. doi: 10.3321/j.issn:1001-4861.2008.11.010SHAO Chang-li, YIN Chang-long, ZHANG Jun-ping, CHEN Shi-an, LIU Chen-fuang. Synthesis of mesoporous Ni-Mo mixed oxides with urea method[J]. Chin J Inorg Chem, 2008, 24(11):1782-1788. doi: 10.3321/j.issn:1001-4861.2008.11.010 [16] 张乐, 龙湘云, 刘学芬, 刘清河, 陈若雷.黏结剂对Ni-Mo-W体相加氢精制催化剂性能的影响[J].石油学报(石油加工), 2012, 28(1):7-14. http://mall.cnki.net/magazine/Article/SXJG201201003.htmZHANG Le, LONG Xiang-yun, LIU Xue-fen, LIU Qing-he, CHEN Ruo-lei. Influence of binders on the properties and performance of Ni-Mo-W bulk hydrotreating catalysts[J]. Acta Pet Sin (Pet Process Sect) 2012, 28(1):7-14. http://mall.cnki.net/magazine/Article/SXJG201201003.htm [17] 赵蕾艳, 殷长龙, 李贺, 翟西平, 白振江, 刘晨光. 硅藻土分散的非负载型NiMoW加氢催化剂的研究[C]//第十届全国工业催化技术及应用年会论文集. 太原, 2013: 167-169.ZHAO Yan-lei, YIN Chang-long, LI He, ZAI Xi-ping, BAI Zheng-jiang, LIU Chen-guang. Study on dispersion supported non supported NiMoW hydrogenation catalyst for diatomite[C]//Proceedings of the 10th annual meeting of national industrial catalytic technology and Applications. Taiyuan, 2013: 167-169. [18] LIU D, LI G.C, LIU L H, LIU C G. Synthesis, characterization and hydrodesulfurization activity of silica-dispersed NiMoW trimetallic catalysts[J]. Nat Gas Chem, 2010, 19(9):530-533. https://www.sciencedirect.com/science/article/pii/S1003995309601080 [19] 王海涛, 徐学军, 刘东香, 冯小萍.高活性体相加氢精制催化剂的研制[J].现代化工, 2016, 36(11):64-68. http://mall.cnki.net/magazine/Article/SXJG201201003.htmWANG Hai-tao, XU Xue-jun, LIU Dong-xiang, FENG Xiao-ping. Preparation of high active bulk catalyst for hydrorefining[J]. Mod Chem Ind, 2016, 36(11):64-68. http://mall.cnki.net/magazine/Article/SXJG201201003.htm [20] CHEN Y D, WANG L, LIU X Y, LIU T F, HUANG B K, LI P, JIANG Z X, LI C Hydrodesulfurization of 4, 6-DMDBT on multi-metallic bulk catalyst NiAlZnMoW:Effect of Zn[J]. Appl Catal A:Gen, 2015, 504(9):319-327 https://www.sciencedirect.com/science/article/pii/S0926860X15000642 [21] CHEN Y D, WANG L, ZHANG Y L, LIU T F, LIU X Y, JIANG Z X, LI C. A new multi-metallic bulk catalyst with high hydrodesulfurization activity of 4, 6-DMDBT prepared using layered hydroxide salts as structural templates[J]. Appl Catal A:Gen, 2014, 474:69-77. doi: 10.1016/j.apcata.2013.09.002 [22] 殷长龙, 白振江, 赵蕾艳, 张俊萍, 刘晨光.聚乙二醇对非负载型Ni-Mo催化剂结构及加氢脱硫性能的影响[J].化学工程与技术, 2013, 3(6):208-214. http://www.cnki.com.cn/Article/CJFDTOTAL-XTXB201103010.htmYIN Chang-long, BAI Zheng-jiang, ZHAO Lei-yan, ZHANG Jun-ping, LIU Chen-guang. Effect of PEG on the structure and hydrodesulfurization performance of unsupported Ni-Mo catalyst[J]. J Chem Eng Technol, 2013, 3(6):208-214. http://www.cnki.com.cn/Article/CJFDTOTAL-XTXB201103010.htm [23] HUIRACHE ACUNA R, ALONSO NUNEZ G, PARAGUAY DELGADO F, LARA ROMERO J BERHAULT G, RIVERA MUNOZ E M. Unsupported trimetallic CoMoW sulfide HDS catalysts prepared by in situ decomposition of sulfur-containing precursors[J]. Catal Today, 2015, 250(15):28-37. https://www.sciencedirect.com/science/article/pii/S0920586114004969 [24] LIU D, LI G.C, LIU L H, LIU C G. The synthesis of bulk Ni-Mo-W hydrodesulphurization catalysts with high activity by using methylcellulose[J]. Pet Sci Technol, 2010, 28(14):1485-1491. doi: 10.1080/10916461003627391 [25] LIU H, LIU Q Z, ZHANG J Z, YIN C L, ZHAO Y X, YIN S M, LIU C G, SUN W F. PVP-assisted synthesis of unsupported NiMo catalysts with enhanced hydrodesulfurization activity[J]. Fuel Process Technol, 2017, 160(7):93-101. https://www.sciencedirect.com/science/article/pii/S0378382016310153 [26] HENSENE J M, KOOYMAN P J, VAN DER MEER Y. VAN DER KRAAN A M, DE BEER V H J, BAN VEEN J A R, V AN SANTEN R A. The relation between morphology and hydrotreating activity for supported MoS2 particles[J]. J Catal, 2001, 199(2):224-235. https://www.sciencedirect.com/science/article/pii/S0021951700931580 [27] CHIANELLI R R, DAAGE M, LEDOUX M J. Fundamental studies of transition-metal sulfide catalytic materials[J]. Adv Catal, 1994, 40:177-232. https://www.sciencedirect.com/science/article/pii/S0360056408606586 [28] HEIN J, GUTIERREZ L Y, ALBERSBERGERS, HAN J Y, JENTYS A, LERCHER J A. Towards understanding structure-activity relationships of Ni-Mo-W sulfide hydrotreating Catalysts[J]. ChemCatChem, 2017, 9(4):629-641. doi: 10.1002/cctc.v9.4 [29] BEJ S K, MAITY S K, TURAGA U T. Search for an efficient 4, 6-DMDBT hydrodesulfurization catalyst:A review of recent studies[J]. Energy Fuels, 2004, 18(5):1227-1237. doi: 10.1021/ef030179+ -





 下载:
下载: