Effect of NO on the performance of Cu-Mn spinel sorbent in the removal of Hg0 from flue gas
-
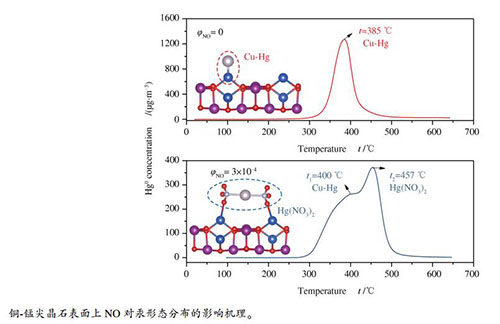
摘要: 采用实验与量子化学计算相结合的方法研究了烟气中NO对铜-锰尖晶石脱汞性能的影响机理。结果表明,NO在高于250 ℃时抑制铜-锰尖晶石对Hg0的脱除,主要归因于NO与Hg0之间的竞争吸附作用;而在温度低于250 ℃时,NO对铜-锰尖晶石的Hg0脱除性能影响较小。吸附剂表面上吸附的汞主要以Cu-Hg合金和Hg(NO3)2的形式存在。铜-锰尖晶石表面上部分NO被氧化成NO2并与吸附态汞反应形成Hg(NO3)2。吸附剂表面上Cu和Mn原子为NO与Hg0的吸附活性位点,NO的吸附能大于Hg0的吸附能;因此,NO与Hg0之间存在竞争吸附。由于Cu、Mn、N原子之间的强烈轨道杂化作用,NO与铜-锰尖晶石吸附剂表面之间具有较强的相互作用。Abstract: The effect of NO on the performance of Cu-Mn spinel sorbent in the removal of Hg0 from flue gas was investigated by using experimental and density functional theory (DFT) calculation methods. The results indicate that NO shows slightly inhibitory effect on the Hg0 removal on the CuMn2O4 sorbent at high temperature (>250 ℃), probably due to the competitive adsorption between NO and Hg0 on the sorbent surface. At low temperature (< 250 ℃), NO has little influence on the Hg0 removal. The mercury species adsorbed on the CuMn2O4 sorbent exist mainly in the forms of Cu-Hg amalgam and Hg(NO3)2. NO can be oxidized into NO2 over the CuMn2O4 sorbent and NO2 then react with adsorbed mercury to form Hg(NO3)2. Cu and Mn atoms are identified as the active sites for the adsorption of NO and Hg0. There is a strong interaction between NO and CuMn2O4 surface, which is closely associated with the orbital hybridization of Cu, Mn, and N atoms.
-
Key words:
- Hg0 adsorption /
- Cu-Mn spinel /
- NO /
- adsorption mechanism /
- quantum chemistry calculation
-
图 5 NO在CuMn2O4表面上稳定吸附结构的原子投影态密度
Figure 5 Density of states (DOS) of NO adsorption on the CuMn2O4 surface
(a): DOS of Cu atom during NO adsorption on Cu site; (b): DOS of Mn atom during NO adsorption on Mn site; (c): DOS of N atom during NO adsorption on Cu site; (d): DOS of N atom during NO adsorption on Mn site
-
[1] YANG Y J, LIU J, WANG Z. Reaction mechanisms and chemical kinetics of mercury transformation during coal combustion[J]. Prog Energy Combust Sci, 2020, 79: 100844. [2] ZHAO S L, PUDASAINEE D, DUAN Y F, GUPTA R, LIU M, LU J H. A review on mercury in coal combustion process: Content and occurrence forms in coal, transformation, sampling methods, emission and control technologies[J]. Prog Energy Combust Sci, 2019, 73: 26-64. [3] YANG Y J, MIAO S, LIU J, WANG Z, YU Y N. Cost-effective manganese ore sorbent for elemental mercury removal from flue gas[J]. Environ Sci Technol, 2019, 53(16): 9957-9965. [4] YAO T, DUAN Y F, BISSON T M, GUPTA R, PUDASAINEE D, ZHU C, XU Z H. Inherent thermal regeneration performance of different MnO2 crystallographic structures for mercury removal[J]. J Hazard Mater, 2019, 374: 267-275. [5] ZHENG Y J, JENSEN A D, WINDELIN C, JENSEN F. Review of technologies for mercury removal from flue gas from cement production processes[J]. Prog Energy Combust Sci, 2012, 38(5): 599-629. [6] KANG M, PARK E D, KIM J M, YIE J E. Cu-Mn mixed oxides for low temperature NO reduction with NH3[J]. Catal Today, 2006, 111(3): 236-241. [7] LIU P, HE H P, WEI G L, LIANG X L, QI F H, TAN F D, TAN W, ZHU J X, ZHU R L. Effect of Mn substitution on the promoted formaldehyde oxidation over spinel ferrite: Catalyst characterization, performance and reaction mechanism[J]. Appl Catal B: Environ, 2016, 182: 476-484. [8] YANG Y J, LIU J, WANG Z, LONG Y, DING J Y. Interface reaction activity of recyclable and regenerable Cu-Mn spinel-type sorbent for Hg0 capture from flue gas[J]. Chem Eng J, 2019, 372: 697-707. [9] YANG Y J, LIU J, WANG Z, ZHANG Z, DING J Y, YU Y N. Nature of active sites and an oxygen-assisted reaction mechanism for mercury capture by spinel-type CuMn2O4 sorbents[J]. Energy Fuels, 2019, 33(9): 8920-8926. [10] YANG Y J, LIU J, LIU F, WANG Z, DING J Y, HUANG H. Reaction mechanism for NH3-SCR of NOx over CuMn2O4 catalyst[J]. Chem Eng J, 2019, 361: 578-587. [11] 杨应举, 张保华, 刘晶, 王震, 苗森.可再生循环利用CuxMn(3-x)O4尖晶石吸附剂脱汞性能[J].燃烧科学与技术, 2017, 23(6): 511-515.YANG Ying-ju, ZHANG Bao-hua, LIU Jing, WANG Zhen, MIAO Sen. Mercury removal by recyclable and regenerable CuxMn(3-x)O4 spinel-type sorbents[J]. J Combust Sci Technol, 2017, 23(6): 511-515. [12] RUMAYOR M, DIAZ-SOMOANO M, LOPEZ-ANTON M. Mercury compounds characterization by thermal desorption[J]. Talanta, 2013, 114: 318-322. -





 下载:
下载: