Influence of H2O on the adsorption of SO2 on CaO (001) surface: A DFT study
-
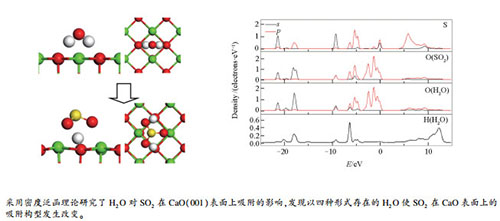
摘要: 采用密度泛函理论研究了H2O对SO2在CaO(001)表面上吸附的影响。结果表明,以四种形式(-H2O、-H、-OH和-H&-OH)存在的H2O使SO2在CaO表面上的吸附构型发生改变。SO2在不同形式H2O基团邻位吸附时,-H使S原子的p轨道态密度峰明显左移且吸附能比洁净表面大90 kJ/mol,其余基团表面吸附能无明显变化;SO2吸附于-OH和-H&-OH生成HSO3基团,吸附能相比于洁净表面较小,可能作为暂态结构;SO2吸附于-H2O生成SO3基团,H2O断键生成的H基团起主要吸附作用,CaO表面上生成类似Ca(OH)2的局部结构且吸附能比洁净表面大45 kJ/mol。
-
关键词:
- 密度泛函理论 /
- H2O /
- SO2 /
- CaO(001)表面 /
- 吸附
Abstract: The influence of H2O on the adsorption of SO2 on CaO (001) surface was investigated by density functional theory (DFT). The results indicate that H2O can have an effect on the adsorption geometries for SO2 on the CaO (001) surface. When SO2 is adsorbed to the CaO surface near a water group of different forms (viz., -H2O, -H, -OH and -H & -OH), the H group makes the adsorption energy 90 kJ/mol higher with the sulfur p-orbital shifting downward, whereas other groups have little effect on the adsorption energy. When SO2 is adsorbs to the -OH surface and -H & -OH surface, bisulfite-like structures are formed, with lower adsorption energies and tending to form more stable structures as intermediates. When SO2 adsorbs to the -H2O surface, bisulfite-like structure is formed and the H2O group decomposes to Ca(OH)2 like-structure on the CaO surface; the new H groups mainly bond the bisulfite and make adsorption energy 45 kJ/mol higher.-
Key words:
- density functional theory /
- H2O /
- SO2 /
- CaO(001) surface /
- adsorption
-
表 1 SO2在不同CaO表面吸附构型成键参数及电荷转移
Table 1 Adsorption energy, S-O bond distance, O-S-O bond angel, S-Osurf distance and charge transfer to SO2 for SO2 adsorption on different CaO surfaces
Structure Adsorption energy /(kJ·mol-1) S-O bond distance /nm O-S-O bond angle /(°) S-Osurf distance /nm Charge transfer to SO2 (e) 1-a 163.99 0.152 111.00 0.172 -0.27 1-b 172.08 0.150 111.00 0.169 -0.26 3-a 157.34 0.198 140.75 0.212 -0.27 3-b 147.99 0.150 110.78 0.174 -0.27 3-c 159.34 0.151 110.53 0.168 -0.27 5-a 165.43 0.151 110.58 0.167 -0.27 5-b 170.02 0.150 110.16 0.171 -0.27 7-a 160.62 0.150 110.44 0.168 -0.26 7-b 156.66 0.150 110.44 0.171 -0.26 8-a 259.92 0.154 110.36 - -0.66 8-b 252.14 0.153 112.72 - -0.67 8-c 229.63 0.153 111.80 0.299 -0.67 8-d 251.95 0.154 112.14 - -0.67 表 2 不同情况吸附构型化学键布居数对比
Table 2 Bond populations for SO2 adsorption on different surfaces
Structure Bond Population Bond distance Atomic position SO2 molecule O1-S1 0.33 1.44 O2-S1 0.33 1.44 SO2 adsorbing on clean CaO surfaces (1-a) O1-S1 0.39 1.50 O1, O2 from SO 2,O3 from CaO surface O2-S1 0.39 1.50 O3-S1 0.19 1.71 SO2 adsorbing on -H2O surface (4) H1-O4 0.48 0.98 O4 and O5 from CaO surface, O3 from H2O, O1 and O2 from SO2 H2-O5 0.59 0.98 O1-S1 0.46 1.46 O2-S1 0.33 1.50 O3-S1 0.11 1.85 SO2 adsorbing on -OH surface (7-c) O3-S1 0.48 0.99 O1 from OH group,O2 and O3 from SO2 O3-S1 0.45 1.47 O2-S1 0.37 1.50 O1- S1 0.13 1.81 O2-Ca1 0.11 2.42 -
[1] 邹耀民, 杨义文.大气污染物二氧化硫的荧光检测技术研究进展[J].上海化工, 2019, 44(4): 39-43. doi: 10.3969/j.issn.1004-017X.2019.04.014ZOU Yao-min, YANG Yi-wen. Advances in the fluorescence detection technology of atmospheric pollutant sulfur dioxide[J]. Shanghai Chem Ind, 2019, 44(4): 39-43. doi: 10.3969/j.issn.1004-017X.2019.04.014 [2] 中华人民共和国国家发展和改革委员会.煤电节能减排升级与改造行动计划(2014-2020年)[EB /OL]. http://www.ndrc.gov.cn/gzdt/201409/t20140919_626240.html.National Development and Reform Commission. Coal-fired energy-saving emission reduction upgrade plan(2014-2020)[EB /OL]. http://www.ndrc.gov.cn/gzdt/201409/t20140919_626240.html. [3] 蒋敏华, 肖平.大型循环流化床锅炉技术[M].北京:中国电力出版社, 2009.JIANG Min-hua, XIAO Ping. Large-Scale Circulating Fluidized Bed Boiler Technology[M]. Beijing: China Electric Power Press, 2009. [4] WANG C, ZHANG Y, JIA L, TAN Y. Effect of water vapor on the pore structure and sulfation of CaO[J]. Fuel, 2014, 130: 60-65. doi: 10.1016/j.fuel.2014.04.007 [5] 姜中孝, 段伦博, 陈晓平, 赵长遂.空气燃烧与O2/CO2燃烧气氛下水蒸气对石灰石煅烧/硫化特性的影响[J].中国电机工程学报, 2013, 33(26): 14-20. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgdjgcxb201326019JIANG Zhong-xiao, DUAN Lun-bo, CHEN Xiao-ping, ZHAO Chang-sui. Effect of water vapor on indirect sulfation during air and O2/CO2 combustion[J]. Proc CSEE, 2013, 33(26): 14-20. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgdjgcxb201326019 [6] STEWART M C, MANOVIC V, ANTHONY E J, MACCHI A. Enhancement of indirect sulphation of limestone by steam addition[J]. Environ Sci Technol, 2010, 44(22): 8781-8786. doi: 10.1021/es1021153 [7] HSIA C, PIERRE G R ST, RAGHUNATHAN K, FAN L S. Diffusion through CaSO4 formed during the reaction of CaO with SO2 and O2[J]. AIChE J, 1993, 39(4): 698-700. doi: 10.1002/aic.690390419 [8] HSIA C, PIERRE G R S, FAN L. Isotope study on diffusion in CaSO4 formed during sorbent-flue-gas reaction[J]. AIChE J, 1995, 41(10): 2337-2340. doi: 10.1002/aic.690411020 [9] WANG C, JIA L, TAN Y, ANTHONY E J. The effect of water on the sulphation of limestone[J]. Fuel, 2010, 89(9): 2628-2632. doi: 10.1016/j.fuel.2010.04.022 [10] 王世昌, 徐旭常, 姚强.水蒸汽对CaO颗粒脱硫反应催化作用的实验研究[J].中国电机工程学报, 2004, 24(9): 256-260. http://d.old.wanfangdata.com.cn/Periodical/zgdjgcxb200409045WANG Shi-chang, XU Xu-chang, YAO Qiang. Experimental study on the catalysis effect of steam in the dry flue gas desulfurization reaction by CaO particles[J]. Proc CSEE, 2004, 24(9): 256-260. http://d.old.wanfangdata.com.cn/Periodical/zgdjgcxb200409045 [11] 祁海鹰, 由长福, 王爱军, 徐旭常.蒸汽活化改善中温烟气脱硫的机理[J].中国电机工程学报, 2002, 22(7): 119-124. doi: 10.3321/j.issn:0258-8013.2002.07.025QI Hai-ying, YOU Zhang-fu, WANG Ai-jun, XU Xu-chang. Mechanism of improving the midiem temperature FGD process by reactivating sobernts by steam[J]. Proc CSEE, 2002, 22(7): 119-124. doi: 10.3321/j.issn:0258-8013.2002.07.025 [12] ZHANG B, LIU J, SHEN F. Heterogeneous mercury oxidation by HCl over CeO2 catalyst: Density Functional theory study[J]. J Phys Chem C, 2015, 119(27): 15047-15055. doi: 10.1021/acs.jpcc.5b00645 [13] CHENG L, LI W, CHEN Z, AI J, ZHOU Z, LIU J. DFT study of oxygen adsorption on Mo2C(001) and (201) surfaces at different conditions[J]. Appl Surf Sci, 2017, 411: 394-399. doi: 10.1016/j.apsusc.2017.03.195 [14] LENTZ C, JAND S P, MELKE J, ROTH C, KAGHAZCHI P. DRIFTS study of CO adsorption on Pt nanoparticles supported by DFT calculations[J]. J Mol Catal A: Chem, 2017, 426: 1-9. doi: 10.1016/j.molcata.2016.10.002 [15] 董静兰, 耿晓, 高正阳, 刘彦丰.飞灰中的缺陷位SiO2对痕量元素As的吸附机理[J].燃料化学学报, 2018, 46(11): 1401-1408. doi: 10.3969/j.issn.0253-2409.2018.11.015DONG Jing-lan, GENG Xiao, GAO Zheng-yang, LIU Yan-feng.Adsorption mechanism of trace As on the defect sites of SiO2 in fly ash[J]. J Fuel Chem Technol, 2018, 46(11): 1401-1408. doi: 10.3969/j.issn.0253-2409.2018.11.015 [16] 刘磊, 金晶, 林郁郁, 侯封校.钙元素对焦炭表面NO吸附行为的影响:密度泛函理论研究[J].燃料化学学报, 2015, 43(12): 1414-1419. doi: 10.3969/j.issn.0253-2409.2015.12.002LIU Lei, JIN Jing, LIN Yu-yu, HOU Feng-xiao. Effect of calcium on the absorption of NO on char surface: A density functional theory study[J]. J Fuel Chem Technol, 2015, 43(12): 1414-1419. doi: 10.3969/j.issn.0253-2409.2015.12.002 [17] GALLOWAY B, PADAK B. Effect of flue gas components on the adsorption of sulfur oxides on CaO(100)[J]. Fuel, 2017, 197: 541-550. doi: 10.1016/j.fuel.2017.02.057 [18] SASMAZ E, WILCOX J. Mercury species and SO2 adsorption on CaO(100)[J]. J Phys Chem C, 2008, 112(42): 16484-16490. doi: 10.1021/jp801250h [19] WANG G, WANG W, FAN L, LI Y. CO2 and SO2 sorption on the alkali metals doped CaO(100)surface: A DFT-D study[J]. Appl Surf Sci, 2017, 425: 972-977. doi: 10.1016/j.apsusc.2017.07.158 [20] CLARK S J, SEGALL M D, PICKARD C J, HASNIP P J, PROBERT M I J, REFSON K, PAYNE M C. First principles methods using CASTEP[J]. Z Krist-Cryst mater, 2005, 220(5/6). http://d.old.wanfangdata.com.cn/NSTLQK/NSTL_QKJJ027843518/ [21] SEGALL M D, LINDAN P J D, PROBERT M J, PICKARD C J, HASNIP P J, CLARK S J, PAYNE M C. First-principles simulation: Ideas, illustrations and the CASTEP code[J]. J Phys: Condens matter, 2002, 14(11): 2717-2744. doi: 10.1088/0953-8984/14/11/301 [22] PERDEW J P, CHEVARY J A, VOSKO S H, JACKSON K A, PEDERSON M R, SINGH D J, FIOLHAIS C. Erratum: Atoms, molecules, solids, and surfaces: Applications of the generalized gradient approximation for exchange and correlation[J]. Phys Rev B, 1993, 48: 4978. http://d.old.wanfangdata.com.cn/NSTLQK/NSTL_QKJJ0214985123/ [23] PERDEW J P, BURKE K, ERNZERHOF M. Generalized gradient approximation made simple[J]. Phys Rev Lett, 1996, 77(18): 3865-3868. doi: 10.1103/PhysRevLett.77.3865 [24] ZINTL E, HARDER A, DAUTH B, ELEKTROCHEM Z. Angew. Zeitschrift für anorganische und allgemeine Chemie[J]. Phys Chem, 1934, 40: 588. [25] CUNNINGHAM T L P COOPER D, GERRATT J, KARADAKOV P B, RAIMONDI M. Chemical bonding in oxofluorides of hypercoordinate sulfur[J]. J Chem Soc Faraday Trans, 1997, 93: 2247-2254. doi: 10.1039/a700708f [26] FAN Y, ZHUO Y, LI L. SeO2 adsorption on CaO surface: DFT and experimental study on the adsorption of multiple SeO2 molecules[J]. Appl Surf Sci, 2017, 420: 465-471. doi: 10.1016/j.apsusc.2017.04.233 [27] FAN Y, ZHUO Y, ZHU Z, LI L, CHEN Q, LOU Y. Density functional theory study on Hg removal mechanisms of Cu-impregnated activated carbon prepared by simplified method[J]. Korean J Chem Eng, 2016, 33(10): 2869-2877. doi: 10.1007/s11814-016-0153-z [28] DE LEEUW N H, WATSON G W, PARKER S C. Atomistic simulation of the effect of dissociative adsorption of water on the surface structure and stability of calcium and magnesium oxide[J]. J Phys Chem C, 1995, 99(47): 17219-17225. doi: 10.1021/j100047a028 [29] DE LEEUW N H, PURTON J A, PARKER S C, WATSON G W, KRESSE G. Density functional theory calculations of adsorption of water at calcium oxide and calcium fluoride surfaces[J]. Surf Sci, 2000, 452(1/3): 9-19. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=800824640274aa24002a06848c26634e [30] CARRASCO J, ILLAS F, LOPEZ N. Dynamic ion pairs in the adsorption of isolated water molecules on alkaline-earth oxide (001) surfaces[J]. Phys Rev Lett, 2008, 100(1): 16101. doi: 10.1103/PhysRevLett.100.016101 [31] FAN Y, YAO J G, ZHANG Z, SCEATS M, ZHUO Y, LI L, MAITLAND G C, FENNELL P S. Pressurized calcium looping in the presence of steam in a spout-fluidized-bed reactor with DFT analysis[J]. Fuel Process Technol, 2018, 169: 24-41. doi: 10.1016/j.fuproc.2017.09.006 -





 下载:
下载: