Comparison of reaction mechanism of thiophene hydrodesulfurization on Au13 and Pt13 clusters
-
摘要: 采用密度泛函理论研究了噻吩在立方正八面体的M13(M=Au、Pt)团簇上的吸附和加氢脱硫行为。结果表明,噻吩以环吸附于Au13上的Hol-tri位或Pt13上的Hol-quadr位时最稳定,且Pt13上的吸附稳定性更高。在M13催化体系中,按间接加氢脱硫机理,反应可能依顺式加氢的方式进行;其中,C-S键断裂开环所需的活化能最高,是反应的限速步骤;按直接加氢脱硫机理,HS加氢所需活化能最高,是反应的限速步骤。同时该机理总体所需活化能较间接加氢脱硫机理更低,是更为合理的脱硫机理。噻吩加氢脱硫过程中,Au13体系为放热反应,而Pt13体系为吸热反应,并且Au13体系加氢所需活化能更低;因此,Au13更有利于噻吩加氢脱硫反应的进行。Abstract: The behaviors of thiophene adsorption and hydrodesulfurization on cubic octahedral M13 (M=Au, Pt) clusters were investigated by density functional theory. The results show that the adsorption energy of thiophene on Pt13 is higher than that on Au13; on the Au13 cluster, the Hol-tri site is most stable for the thiophene adsorption with ring, whereas on the Pt13 cluster, the Hol-quadr site is most stable. By the indirect desulfurization mechanism, the desulfurization is achieved probably via the cis-hydrogenation; the removal of C-S is the rate-determining step. By the direct desulfurization mechanism, the HS hydrogenation turns to be the rate-determining step. The desulfurization is most likely via the direct desulfurization mechanism, which exhibits much lower activation energy than the indirect desulfurization mechanism. The energy change for thiophene desulfurization on the Au13 cluster is exothermic, whereas on the Pt13 cluster it is endothermic; as a result, the hydrodesulfurization on Au13 is much easier than that on Pt13.
-
Key words:
- thiophene /
- hydrodesulfurization /
- adsorption /
- Au13 cluster /
- Pt13 cluster /
- density functional theory
-
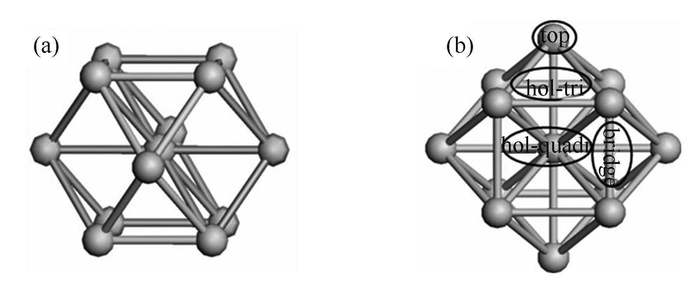
表 1 噻吩在M13上的吸附能
Table 1 Adsorption energies of thiophene on M13
M13 Eads/(kJ·mol-1) top bridge hol-tri hol-quadr Au13 -76.5 -80.4 -89.9 -66.6 Pt13 -98.5 -194.0 -287.8 -300.0 表 2 噻吩在M13上稳定吸附构型的吸附能和结构参数
Table 2 Adsorption energies and structure parameters of thiophene on M13
Model Eads/(kJ·mol-1) d1/nm d2/nm d3/nm d4/nm d5/nm Thiophene (simulation) - 0.172 9 0.137 6 0.142 4 0.137 6 0.172 9 Thiophene/Au13 -89.9 0.183 5 0.145 2 0.137 5 0.145 1 0.183 0 Thiophene/Pt13 -300.0 0.186 8 0.148 2 0.143 8 0.148 2 0.187 0 Δd/(Au13) - 0.010 6 0.007 6 -0.004 9 0.007 5 0.010 1 Δd/(Pt13) - 0.013 9 0.010 6 0.001 4 0.010 6 0.014 1 表 3 噻吩在M13上的稳定吸附构型的Mulliken电荷布居
Table 3 Mulliken charges of thiophene at preferential advantage adsorption site on M13
Atom Charge /e S C1 C2 C3 C4 H1 H2 H3 H4 tol Thiophene/Au13 0.029 -0.032 0.009 0.009 -0.039 0.170 0.115 0.115 0.170 0.537 Thiophene/Pt13 0.145 0.053 0.044 0.045 0.053 0.154 0.136 0.136 0.154 0.920 表 4 间接脱硫各反应在M13上的活化能和反应能量变化
Table 4 Activation barriers and reaction energy of indirect desulfurization reaction on M13
Step Au13/(kJ·mol-1) Pt13/(kJ·mol-1) reactant product ΔE Ea reactant product ΔE Ea H2 H -12.4 69.8 H2 H -99.6 5.0 (1) C4H4S α-C4H5S -33.7 16.0 C4H4S α-C4H5S 135.0 138.8 (2) C4H4S β-C4H5S -75.6 129.2 C4H4S β-C4H5S 44.2 138.1 (3) α-C4H5S α, α-C4H6S -34.6 100.8 β-C4H5S β, α-C4H6S 81.7 116.8 (4) α-C4H5S α, β-C4H6S -38.8 99.7 - - - - (5) α, β-C4H6S α, β, α-C4H7S -68.5 48.6 β, α-C4H6S β, α, α-C4H7S 18.6 166.0 (6) α, β-C4H6S α, β, β-C4H7S 25.1 33.1 β, α-C4H6S β, α, β-C4H7S 12.8 126.2 (7) α, β, β-C4H7S C4H8S -155.6 96.1 β, α, β-C4H7S C4H8S 68.2 123.9 (8) C4H8S C4H9S -9.0 227.9 C4H8S C4H9S 40.9 220.4 (9) C4H9S C4H10+S -148.0 111.5 C4H9S C4H10+S -20.6 149.7 (10) S HS 44.2 46.6 S HS 81.9 104.2 (11) HS H2S 43.2 80.3 HS H2S 150.7 166.6 表 5 直接脱硫各反应在M13上的活化能和反应能量变化
Table 5 Activation barriers and reaction energy of direct desulfurization reaction on M13
Step Reaction Au13 Pt13 ΔE/(kJ·mol-1) Ea/(kJ·mol-1) ΔE/(kJ·mol-1) Ea/(kJ·mol-1) (1′) C4H4S+H→C4H5S -88.8 54.7 92.2 151.4 (2′) C4H5S+H→C4H6+S -5.3 22.2 -190.8 137.1 (3′) C4H6+S+H→C4H6+HS -16.6 45.9 9.0 19.8 (4′) C4H6+HS+H→C4H6+H2S -18.5 82.9 150.5 154.8 -
[1] BASTON E P, FRANCA A B, NETO A V D, URQUIETA-GONZALEZ E A.Incorporation of the precursors of Mo and Ni oxides directly into the reaction mixture of sol-gel prepared gamma-Al2O3-ZrO2 supports-Evaluation of the sulfided catalysts in the thiophene hydrodesulfurization[J].Catal Today, 2015, 246:184-190. doi: 10.1016/j.cattod.2014.10.035 [2] LIAO C N, WANG J Y, LI B.Mechanism of Mo-catalyzed C-S cleavage of thiophene[J].J Organomet Chem, 2014, 749:275-286. doi: 10.1016/j.jorganchem.2013.10.013 [3] 祖运, 秦玉才, 高雄厚, 莫周胜, 张磊, 张晓彤, 宋丽娟.催化裂化条件下噻吩与改性Y分子筛的作用机制[J].燃料化学学报, 2015, 43(7):862-869. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18664.shtmlZU Yun, QIN Yu-cai, GAO Xiong-hou, MO Zhou-sheng, ZHANG Lei, ZHANG Xiao-tong, SONG Li-juan.Mechanisms of thiophene conversion over the modified Y zeolites under catalytic cracking conditions[J].J Fuel Chem Technol, 2015, 43(7):862-869. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18664.shtml [4] 刘理华, 刘书群, 尹海亮, 柳云骐, 刘晨光.Ni2P和MoS2催化剂在二苯并噻吩加氢脱硫反应中的氢溢流效应[J].燃料化学学报, 2015, 43(6):708-713. doi: 10.1016/S1872-5813(15)30022-0LIU Li-hua, LIU Shu-qun, YIN Hai-liang, LIU Yun-qi, LIU Chen-guang.Hydrogen spillover effect between Ni2P and MoS2 catalysts in hydrodesulfurization of dibenzothiophene[J].J Fuel Chem Technol, 2015, 43(6):708-713. doi: 10.1016/S1872-5813(15)30022-0 [5] SHAN J, TENHU H.Recent advances in polymer protected gold nanoparticles:Synthesis, properties and applications[J].Chem Commun, 2007, 44:4580-4598. [6] CORMA A, SERNA P.Chemoselective hydrogenation of nitro compounds with supported gold catalysts[J].Science, 2006, 313(5785):332-334. doi: 10.1126/science.1128383 [7] LI X H, ZHENG W L, PAN H Y, YU Y, CHEN L, WU P.Pt nanoparticles supported on highly dispersed TiO2 coated on SBA-15 as an efficient and recyclable catalyst for liquid-phase hydrogenation[J].J Catal, 2013, 300:9-19. doi: 10.1016/j.jcat.2012.12.007 [8] RAMOS-FERNANDEZ E V, PIETERS C, VAN DER LINDEN B, JNAN-ALCANIZ J, SERRA-CRESPO P, VERHOEVEN M W G M, NIEMANTSVERDRIET H, GASCON J, KAPTEIJN F.Highly dispersed platinum in metal organic framework NH2-MIL-101(Al) containing phosphotungstic acid-Characterization and catalytic performance[J].J Catal, 2012, 289:42-52. doi: 10.1016/j.jcat.2012.01.013 [9] LUO S R, CHEN S Z, HSU Y H, YAU S L, LIN Y J, HUANG P Y, CHEN M C.In situ scanning tunneling microscopy characterization of thienothiophene-based semiconducting organic molecules adsorbed on a Au (111) electrode[J].Surf Sci, 2013, 616:155-160. doi: 10.1016/j.susc.2013.05.013 [10] WANG H M, LGLESIA E.Mechanism and site requirements of thiophene hydrodesulfurization catalyzed by supported Pt clusters[J].Chemcatchem, 2011, 3(7):1166-1175. doi: 10.1002/cctc.v3.7 [11] ZHU H Y, GUO W Y, JIANG R B, ZHAO L M, LU X Q, LI M, FU D L, SHAN H H.Decomposition of methanthiol on Pt (111):A density functional investigation[J].Langmuir, 2010, 26(14):12017-12025. doi: 10.1021/la101678d [12] 倪哲明, 施炜, 夏明玉, 薛继龙.Au (111) 面上噻吩加氢脱硫反应机理的理论研究[J].高等学校化学学报, 2013, 34(10):2353-2362. http://www.cnki.com.cn/Article/CJFDTOTAL-GDXH201310020.htmNI Zhe-ming, SHI Wei, XIA Ming-yu, XUE Ji-long.Theoretical studies on reaction mechanism of hydrodesulfurization of thiophene catalyzed by Au (111) plane[J].Chem J Chin Univ, 2013, 34(10):2353-2362. http://www.cnki.com.cn/Article/CJFDTOTAL-GDXH201310020.htm [13] LI Z, CHEN Z X, HE X, KANG G J.Theoretical studies of acrolein hydrogenation on Au-20 nanoparticle[J].J Chem Phys, 2010, 132(18):184702. doi: 10.1063/1.3407439 [14] IMADA Y, OSAKI M, NOGUCHI M, MAEDA T, FUJIKI M, KAWAMORITA S, KOMIYA N, NAOTA T.Flavin-functionalized gold nanoparticles as an efficient catalyst for aerobic organic transformations[J].ChemCatChem, 2015, 7(1):99-106. doi: 10.1002/cctc.v7.1 [15] BUCHWALTER P, ROSE J, BRAUNSTEIN P.Multimetallic catalysis based on heterometallic complexes and clusters[J].Chem Rev, 2015, 115(1):28-126. doi: 10.1021/cr500208k [16] LARSSON J A, NOLAN M, GREER J C.Interactions between thiol molecular linkers and the Au-13 nanoparticle[J].J Phys Chem B, 2002, 106(23):5931-5937. doi: 10.1021/jp014483k [17] 徐坤, 冯杰, 褚绮, 张丽丽, 李文英.噻吩在γ-Mo2N (100) 表面上加氢脱硫反应的密度泛函理论研究[J].物理化学学报, 2014, 30(11):2063-2070.XU Kun, FENG Jie, CHU Qi, ZHANG Li-li, LI Wen-ying.Density function theory study of thiophene hydrodesulfurization onγ-Mo2N (100) surface[J].Acta Phys Chim Sin, 2014, 30(11):2063-2070. [18] HAMMER B, HANSEN L B, NORSKOV J K.Improved adsorption energetics within density-functional theory using revised Perdew-Burke-Ernzerhof functionals[J].Phys Rev B, 1999, 59(11):7413-7421. doi: 10.1103/PhysRevB.59.7413 [19] 蒋军辉, 夏盛杰, 倪哲明, 张连阳.巴豆醛在Au (111) 面上的吸附及选择性加氢机理研究[J].高等学校化学学报, 2016, 37(4):693-700. http://www.cnki.com.cn/Article/CJFDTOTAL-GDXH201604016.htmJIANG Jun-hui, XIA Sheng-jie, NI Zhe-ming, ZHANG Lian-yang.Adsorption and selective hydrogenation mechanism of crotonaldehyde on Au (111) surface[J].Chem J Chin Univ, 2016, 37(4):693-700. http://www.cnki.com.cn/Article/CJFDTOTAL-GDXH201604016.htm [20] 代广珍, 蒋先伟, 徐太龙, 刘琦, 陈军宁, 代月花.密度泛函理论研究氧空位对HFO2晶格结构和电学特性影响[J].物理学报, 2015, 64(3):033101.DAI Guang-zhen, JIANG Xian-wei, XU Tai-long, LIU Qi, CHEN Jun-ning, DAI Yue-hua.Effect of oxygen vacancy on lattice and electronic properties of HFO2 by means of density function theory study[J].Acta Phys Sin, 2015, 64(3):033101. [21] GE Q, JENKINS S J, KING D A.Localisation of adsorbate-induced demagnetisation:CO chemisorbed on Ni{110}[J].Chem Phys Lett, 2000, 327(3/4):125-130. [22] DELLEY B.Fast calculation of electrostatics in crystals and large molecules[J].J Phys Chem, 1996, 100(15):6107-6110. doi: 10.1021/jp952713n [23] GULIAMOV O, FRENKEL A I, MENARD L D, NUZZO R G, KRONIK L.Tangential ligand-induced strain in Icosahedral Au-13[J].J Am Chem Soc, 2007, 129(36):10978. doi: 10.1021/ja0725706 [24] APRA E, FORTUNELLI A.Density-functional calculations on platinum nanoclusters:Pt-13, Pt-38, and Pt-55[J].J Phys Chem A, 2003, 107(16):2934-2942. doi: 10.1021/jp0275793 [25] MAGER-MAURY C, BONNARD G, CHIZALLET C, SAUTET P, RAYBAUD P.H-2-induced reconstruction of supported Pt clusters:Metal-support interaction versus surface hydride[J].ChemCatChem, 2011, 3(1):200-207. doi: 10.1002/cctc.201000324 [26] SHAFAI G, HONG S Y, BERTINO M, RAHMAN T S.Effect of ligands on the geometric and electronic structure of Au-13 clusters[J].J Phys Chem C, 2009, 113(28):12072-12078. doi: 10.1021/jp811200e [27] CHENG P, ZHANG S L, WANG P, HUANG S P, TIAN H P.First-principles investigation of thiophene adsorption on Ni-13 and Zn@Ni-12 nanoclusters[J].Comput Theor Chem, 2013, 1020:136-142. doi: 10.1016/j.comptc.2013.07.044 [28] SHI W, ZHANG L Y, XIA S J, NI Z M.Adsorption of thiophene on M (111)(M=Pd, Pt, Au) surfaces[J].Acta Phys Chim Sin, 2014, 30(12):2249-2255. [29] WANG H M, LGLESIA E.Thiophene hydrodesulfurization catalysis on supported Ru clusters:Mechanism and site requirements for hydrogenation and desulfurization pathways[J].J Catal, 2010, 273(2):245-256. doi: 10.1016/j.jcat.2010.05.019 -





 下载:
下载: