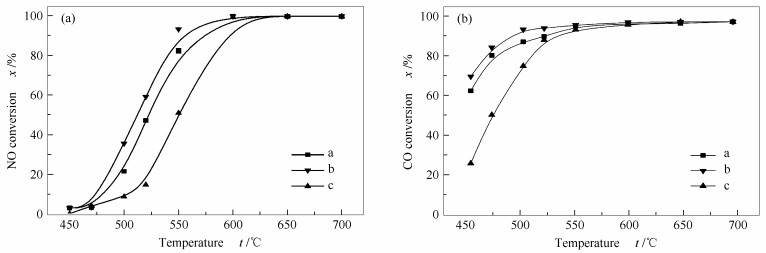
Effect of Mg/Al molar ratios on NO reduction activity of CO using Ce-La/MgAl2O4-x catalysts
-
摘要: 采用初始浸渍法制备了不同Mg/Al物质的量比的Ce-La/MgAl2O4-x催化剂,并通过低温N2-吸附脱附、XRD、H2-TPR和CO-TPR等手段对其进行了表征。结果表明,在Mg/Al物质的量比为0.5时,催化剂催化CO还原NO的性能最好。这主要是因为适量Mg的添加促进了CeO2的分散和Ce-O-La固溶体的形成,从而使得表面Ce3+和氧空穴增加。两者的协同作用使得Ce-La/MgAl2O4-0.5表现出最佳的催化性能。另外,适量Mg的引入可以抑制Ce(SO4)2和Ce2(SO4)3的形成,从而提高了Ce-La/MgAl2O4-0.5催化剂抗硫中毒能力。
-
关键词:
- NO还原 /
- Ce-La/MgAl2O4-x /
- Ce-O-La固溶体 /
- 抗硫中毒能力
Abstract: A series of Ce-La/MgAl2O4-x catalysts were prepared by the incipient wetness impregnation method and characterized by BET, XRD, H2-TPR, CO-TPR and in situ FT-IR. The results demonstrate that the catalyst with a Mg/Al molar ratio of 0.5 yields the most uniform dispersion of CeO2 and greatly enhances formation of Ce-O-La solid solution, resulting in the increase of oxygen vacancy and surface Ce3+ content. Thereby, the synergistic effect between surface Ce3+ and oxygen vacancy gives rise to the best catalytic performance of NO reduction. Moreover, introduction of Mg species suppresses tranformation of CeO2 to Ce(SO4)2/Ce2(SO4)3 and then improves the SO2 resistance performance of Ce-La/MgAl2O4-0.5.-
Key words:
- NO reduction /
- Ce-La/MgAl2O4-x catalysts /
- Ce-O-La solid solution /
- SO2 resistance
-
Figure 3 Regeneration ability of poisoned Ce-La/MgAl2O4-0.5 catalyst
■, □, : fresh Ce-La/MgAl2O4-0.5; ●, ○, ⊗: regenerated Ce-La/MgAl2O4-0.5 NO conversion (● and ■), CO conversion (⊗ and ), SO2 conversion( and ) reaction conditions: 0.1%NO, 4%CO, 2%O2, 0.08%SO2, in balance N2, GHSV=24 000 h-1, t=700 ℃ regeneration conditions: 10 mL/min of H2 for 20 min, t=550 ℃
Table 1 Textural properties of the prepared supports and catalysts

Table 2 Textural properties of poisoned catalysts

-
[1] CRUTZEN P J, BRVHL C. Catalysis by NOx as the main cause of the spring to fall stratospheric ozone decline in the northern hemisphere[J]. J Phys Chem A, 2001, 105 : 1579-1582. doi: 10.1021/jp001984h [2] BABICH I V, SESHAN K, LEFFERTS L. Nature of nitrogen specie in coke and their role in NOx, formation during FCC catalyst regeneration[J]. Appl Catal B: Environ, 2005, 59 (3/4): 205-211. [3] SCHMAL M, VANNICE M A, BALDANZA M A S. Pd-xMo/Al2O3 catalysts for NO reduction by CO[J]. J Catal, 1999, 185 : 138-151. doi: 10.1006/jcat.1999.2465 [4] SHIN H K, HIRABAYASHI H, YAHIRO H, WATANABE M. Selective catalytic reduction of no by ethene in excess oxygen over platinum ion-exchanged MFI zeolites[J]. Catal Today, 1995, 26 (1):13-21. doi: 10.1016/0920-5861(95)00125-Y [5] LIMA R K C D, BATISTA M S, WALLAU M, SANCHES E P. High specific surface area LaFeCo perovskites-synthesis by nanocasting and catalytic behavior in the reduction of NO with CO[J]. Appl Catal B: Environ, 2009, 90 (3): 441-450. https://www.researchgate.net/publication/244110671_High_specific_surface_area_LaFeCo_perovskites-Synthesis_by_nanocasting_and_catalytic_behavior_in_the_reduction_of_NO_with_CO [6] ILIEVA L, PANTALEO G, IVANOV I, VENEZIA A M. Gold catalysts supported on CeO2, and CeO2-Al2O3 for NOx reduction by CO[J]. Appl Catal B: Environ, 2006, 65 (1/2): 101-109. [7] TROVARELLI A, LEITENBURG C D, BOARO M, DOLCETTI G. The utilization of ceria in industrial catalysis[J]. Catal Today, 1999, 50 (2): 353-367. doi: 10.1016/S0920-5861(98)00515-X [8] KIM J R, MYEONG W J, IHM S K. Characteristics of CeO2-ZrO2, mixed oxide prepared by continuous hydrothermal synthesis in supercritical water as support of Rh catalyst for catalytic reduction of NO by CO[J]. J Catal, 2009, 263 (1): 123-133. doi: 10.1016/j.jcat.2009.02.001 [9] BAIDYA T, GUPTA A, DESHPANDEY P A, MADRAS G, HEGDE M S. High oxygen storage capacity and high rates of CO oxidation and NO reduction catalytic properties of Ce1-xSnxO2 and Ce0.78Sn0.2Pd0.02O2-δ [J]. J Phys Chem C, 2009, 113 (10): 4059-4068. doi: 10.1021/jp8060569 [10] CHEN J, ZHU J, ZHAN Y, LIN X, CAI G, WEI K, ZHENG Q. Characterization and catalytic performance of Cu/CeO2 and Cu/MgO-CeO2 catalysts for NO reduction by CO[J]. Appl Catal A: Gen, 2009, 363 (1): 208-215. [11] GAYEN A, BAIDYA T, RAMESH G S, SRIHARI R, HEGDE M S. Design and fabrication of an automated temperature programmed reaction system to evaluate 3-way catalysts Ce1-x-y(La/Y)xPtyO2-δ [J]. J Chem Sci, 2006, 118 (1): 47-55. doi: 10.1007/BF02708765 [12] ILIEVA L, PANTALEO G, IVANOV I, NEDYALKOVA R, VENEZIA A M. NO reduction by CO over gold based on ceria doped by rare earth metals[J]. Catal Today, 2008, 139 (3): 168-173. doi: 10.1016/j.cattod.2008.06.033 [13] BHATTACHARYYA A A, WOLTERMANN G M, JIN S Y, KARCH J A, CORMIER W E. Catalytic SOx abatement: The role of magnesium aluminate spinel in the removal of SOx from fluid catalytic cracking (FCC) flue gas[J]. Ind Eng Chem Res, 1988, 27 (8): 1356-1360. doi: 10.1021/ie00080a004 [14] FORNASARI G, TRIFIRÒF, VACCARI A, PRINETTO F, GHIOTTI G, CENTI G. Novel low temperature NOx, storage-reduction catalysts for diesel light-duty engine emissions based on hydrotalcite compounds[J]. Catal Today, 2002, 75 (1/4): 421-429. [15] BOARO M, GIORDANO F, RECCHIA S, SANTO V D, GIONA M, TROVARELLI A. On the mechanism of fast oxygen storage and release in ceria-zirconia model catalysts[J]. Appl Catal B: Environ, 2004, 52 (3): 225-237. doi: 10.1016/j.apcatb.2004.03.021 [16] LI J, LUO G, CHU Y, WEI F. Experimental and modeling analysis of NO reduction by CO for a FCC regeneration process[J]. Chem Eng J, 2012, 184 (2): 168-175. https://www.deepdyve.com/lp/elsevier/experimental-and-modeling-analysis-of-no-reduction-by-co-for-a-fcc-htkk6r16gP [17] TROVARELLI A, Catalytic properties of ceria and CeO2-containing materials[J]. Catal Rev, 1996, 38 (4): 439-520. doi: 10.1080/01614949608006464 [18] KUMAR P A, REDDY M P, HYUN-SOOK B, PHIL H H. Influence of Mg addition on the catalytic activity of alumina supported Ag for C3H6-SCR of NO[J]. Catal Lett, 2009, 131 (1): 85-97. [19] DAMYANOVA S, PEREZ C A, SCHMAL M, BUENO J M C. Characterization of ceria-coated alumina carrier[J]. Appl Catal A: Gen, 2002, 234 (1/2): 271-282. https://www.deepdyve.com/lp/elsevier/experimental-and-modeling-analysis-of-no-reduction-by-co-for-a-fcc-htkk6r16gP [20] WU L, WIESMANN H J, MOODENBAUGH A R, KLIE R F, ZHU Y, WELCH D O, SUENAGA M. Oxidation state and lattice expansion of CeO2-x nanoparticles as a function of particle size[J]. Phys Rev B, 2004, 69 (12): 125415-125417. doi: 10.1103/PhysRevB.69.125415 [21] SONG Z, LIU W, NISHIGUCHI H. Quantitative analyses of oxygen release/storage and CO2 adsorption on ceria and Pt-Rh/ceria[J]. Catal Commun, 2007, 8 (4): 725-730. doi: 10.1016/j.catcom.2006.08.048 [22] SELLICK D R, ARANDA A, GARCÍA T, LÓPEZ J M, SOLSONA B, MASTRAL A M, MORGAN D J, CARLEY A F, TAYLOR S H. Influence of the preparation method on the activity of ceria zirconia mixed oxides for naphthalene total oxidation[J]. Appl Catal B: Environ, 2013, 132-133 (1): 98-106. [23] ACERBI N, GOLUNSKI S, TSANG S C, DALY H, HARDACRE C, SMITH R, COLLIER P. Promotion of ceria catalysts by precious metals: Changes in nature of the interaction under reducing and oxidizing conditions[J]. J Phys Chem C, 2012, 116 (25): 13569-13583. doi: 10.1021/jp212233u [24] MADIER Y, DESCORME C, GOVIC A M L, DUPREZ D. Oxygen mobility in CeO2 and CexZr1-xO2 compounds: Study by CO transient oxidation and 18O/16O isotopic exchange[J]. J Phys Chem B, 1999, 103 (50): 10999-11006. doi: 10.1021/jp991270a [25] LIU X, ZHOU K, WANG L, WANG B, LI Y, AM J. Oxygen vacancy clusters promoting reducibility and activity of ceria nanorods[J]. J Am Chem Soc, 2016, 131 (9): 3140-3141. doi: 10.1021/ja808433d [26] KANTCHEVA M, SAMARSKAYA O, ILIEVA L, PANTALEO G, VENEZIA A M, ANDREEVA D. In situ FT-IR investigation of the reduction of NO with CO over Au/CeO-Al2O3 catalyst in the presence and absence of H2[J]. Appl Cata B: Environ, 2009, 88 (1/2): 113-126. [27] OH S H, FISHER G B, CARPENTER J E, GOODMAN D W. Comparative kinetic studies of CO+O2 and CO+NO reactions over single crystal and supported rhodium catalysts[J]. J Catal, 1986, 100 (2): 360-376. doi: 10.1016/0021-9517(86)90103-X [28] NAM I S, ELDRIDGE J W, KITTRELL J R. Deactivation of a vanadia-alumina catalyst for nitric oxide reduction by ammonia[J]. Ind Eng Chem Prod Res Dev, 2002, 25 (2): 192-197. [29] XU W, HE H, YU Y. Deactivation of a Ce/TiO2 catalyst by SO2 in the selective catalytic reduction of NO by NH3[J]. J Phys. Chem C, 2016, 113 (11): 4426-4432. [30] WAQIFA M, BAZINA P, SAURA O, LAVALLEY, BLANCHARD G, TOURET O. Study of ceria sulfation[J]. Appl Catal B: Environ, 1997, 11 (2): 193-205. doi: 10.1016/S0926-3373(96)00040-9 [31] LUO T, GORTE R J. Characterization of SO2-poisoned ceria-zirconia mixed oxides[J]. Appl Catal B: Environ, 2004, 53 (2): 77-85. doi: 10.1016/j.apcatb.2004.04.020 [32] DONG W K, NAM K B, HONG S C. The role of ceria on the activity and SO2 resistance of catalysts for the selective catalytic reduction of NOxby NH3[J]. Appl Catal B: Environ, 2015, 166-167 (1): 37-44. -





 下载:
下载: