Liquefaction of thermal extracts from co-thermal dissolution of a sub-bituminous coal with lignin and reusability of Ni-Mo-S/Al2O3 catalyst
-
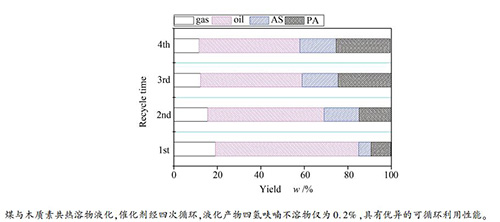
摘要: 对神府次烟煤与木质素共热溶得到的四种具有不同热溶产率的热溶物进行元素分析、红外光谱、同步荧光光谱等表征,对四种热溶物和神府原煤的加氢液化性能进行比较,并进行了催化剂在热溶物液化过程的循环利用性能研究。结果表明,神府煤热溶物较同一温度得到的神府煤与木质素共热溶物具有更多的芳香组分和四环及其以上的多环芳烃。热溶物较神府原煤在液化时具有更高的转化率和油收率。在催化剂Ni-Mo-S/Al2O3作用下,热溶物在液化过程中几乎全部转化,并具有很高的油收率,且神府煤与木质素共热溶物较神府煤热溶物具有更高的油收率。在神府煤与木质素共热溶物的液化过程中,催化剂Ni-Mo-S/Al2O3表现出优异的可循环利用性能,经过四次循环利用后没有观察到催化剂表面的炭沉积现象。Abstract: Four thermal dissolution soluble fractions (TDSFs) with different thermal dissolution soluble yields (TDSYs) obtained from thermal and co-thermal dissolutions (CTDs) of a Chinese sub-bituminous Shenfu (SF) coal and lignin were characterized by elemental analysis, FT-IR and synchronous fluorescence spectrum measurements. The hydro-liquefaction properties of the four TDSFs and SF raw coal with and without catalyst were compared and the recycled use property of the catalyst in hydro-liquefaction of the TDSF from CTD of SF coal and lignin was further probed. The results suggests that the TDSF from the thermal dissolution (TD) of SF coal contained much more amount of aromatic components and polyaromatic hydrocarbons (PAHs) with 4 and more rings than those from the CTD of SF coal and lignin at the same temperature. TDSFs gave much higher liquefaction conversions and oil yields than SF raw coal in hydro-liquefaction with or without catalyst. Almost all TDSF was converted with much high yield of oil and the TDSF from CTD of SF coal and lignin gave higher yield of oil than that from the TD of SF coal in hydro-liquefaction with Ni-Mo-S/Al2O3 catalyst which demonstrated a good reusability in the hydro-liquefaction of TDSF from the CTD of SF coal and lignin. Carbon deposition was hardly observed in the 4 times recycle used catalyst.
-
Key words:
- coal /
- lignin /
- co-thermal dissolution /
- liquefaction /
- catalyst reusability
-
Table 1 Ultimate and proximate analyses of SF coal and lignin
Sample Proximate analysis w/% Ultimate analysis wdaf/% Mad Ad Vdaf C H N S O* SF coal 4.7 15.6 39.3 80.75 5.25 1.18 0.46 12.36 Lignin 7.1 2.9 67.8 63.92 6.31 1.78 0.59 27.40 *:by difference Table 2 Ultimate analysis and atomic ratios of TDSFs
TDSF Ultimate analysis wdaf/% Atomic ratio C H N S Odiff H/C O/C TD-1-MN-360 80.7 5.0 1.6 0.6 12.1 0.74 0.11 CTD-1-MN-360 73.9 5.8 2.2 0.7 17.4 0.95 0.18 CTD-1-MN-320 74.1 5.9 2.1 0.7 17.2 0.95 0.17 CTD-1-MN+ET-320 75.1 6.0 2.0 0.5 16.4 0.95 0.16 Table 3 Hydro-liquefaction of SF raw coal and its TDSFs without catalyst
Sample Yield w/% Conversion x/% gas oil AS PA SF coal 16.4 13.1 11.5 16.0 57.0 TD-1-MN-360 8.2 33.6 24.1 31.1 97.0 CTD-1-MN-360 16.4 28.6 30.3 22.9 98.2 CTD-1-MN-320 21.2 28.1 24.4 24.8 98.5 CTD-1-MN+ET-320 21.6 37.0 21.0 17.3 96.9 Table 4 Hydro-liquefaction of SF raw coal and its TDSFs catalyzed by Ni-Mo-S/Al2O3
Sample Yield w/% Conversion x/% gas oil AS PA SF coal 7.2 27.9 15.1 20.2 70.4 TD-1-MN-360 9.2 52.8 19.2 18.7 99.9 CTD-1-MN-360 9.8 63.7 14.3 12.2 99.8 CTD-1-MN-320 10.8 69.3 10.2 9.0 99.3 CTD-1-MN+ET-320 18.9 65.9 5.8 9.1 99.7 -
[1] SHUI H F, YANG L, SHUI T, PAN C X, LI H P, WANG Z C, LEI Z P, REN S B, KANG S G. Hydro-liquefaction of thermal dissolution soluble fraction of Shenfu subbituminous coal and reusability of catalyst on the hydro-liquefaction[J]. Fuel, 2014, 115(1):227-231. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=b2b0db4a0dd1b34a6627748d9620a072 [2] MIURA K, NAKAGAWA H, ASHIDA R, IHARA T. Production of clean fuels by solvent skimming of coal at around 350℃[J]. Fuel, 2004, 83(6):733-738. doi: 10.1016/j.fuel.2003.09.019 [3] MASAKI K, YOSHIDA T, LI C, TAKANOHASHI T, SAITO I. The effects of pretreatment and the addition of polar compounds on the production of "HyperCoal" from subbituminous coals[J]. Energy Fuels, 2004, 18(4):995-1000. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=CC027660500 [4] KASHIMURA N, TAKANOHASHI T, SAITO I. Effect of noncovalent bonds on the thermal extraction of subbituminous coals[J]. Energy Fuels, 2006, 20(4):1605-1608. doi: 10.1021/ef060050a [5] SHUI H F, ZHOU Y, LI H P, WANG Z C, LEI Z P, REN S B, PAN C X, WANG W W. Thermal dissolution of Shenfu coal in different solvents[J]. Fuel, 2013, 108(6):385-390. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=b0ccdc0e26416003fc55d2d73286a0af [6] YOSHIDA T, LI C, TAKANOHASHI T, MATSUMURA A, SATO S, SAITO I. Effect of extraction condition on "HyperCoal" production (2)-effect of polar solvents under hot filtration[J]. Fuel Process Technol, 2004, 86(1):61-72. doi: 10.1016/j.fuproc.2003.12.003 [7] LU H Y, WEI X Y, YU R, PENG Y L, QI X Z, QIE L M, WEI Q, LV J, ZONG Z M, ZHAO W, ZHAO Y P, NI Z H, WU L. Sequential thermal dissolution of Huolinguole lignite in methanol and in ethanol[J]. Energy Fuels, 2011, 25(6):2741-2745. doi: 10.1021/ef101734f [8] SHUI H F, HUI Z, JIANG Q Q, ZHOU H, PAN C X, WANG Z C, LEI Z P, REN S B, KANG S G. Co-thermal dissolution of Shenmu-Fugu subbituminous coal and sawdust[J]. Fuel Process Technol, 2015, 131(3):87-92. http://www.sciencedirect.com/science/article/pii/S0378382014004846 [9] SHUI H F, MA X Q, YANG L, SHUI T, PAN C X, WANG Z C, LEI Z P, REN S B, KANG S G, XU C. Thermolysis of biomass-related model compounds and its promotion on the thermal dissolution of coal[J]. J Energy Institute, 2017, 90(3):418-423. http://www.sciencedirect.com/science/article/pii/S1743967116300265/pdf?md5=60b24d6f10410953bc432f329cddd0ca&pid=1-s2.0-S1743967116300265-main.pdf [10] COUGHLIN R W, DAVOUDZADEH F. Coliquefaction of lignin and bituminous coal[J]. Fuel, 1986, 65(1):95-106. doi: 10.1016-0016-2361(86)90148-1/ [11] ALTIERI P, COUGHLIN R W. Characterization of products formed during coliquefaction of lignin and bituminous coal at 400℃[J]. Energy Fuels, 1987, 1(3):253-256. doi: 10.1021-ef00003a005/ [12] MATSUMURA Y, NONAKA H, YOKURA H, TSUTSUMI A, YOSHIDA K. Co-liquefaction of coal and cellulose in supercritical water[J]. Fuel, 1999, 78:1049-56. doi: 10.1016/S0016-2361(99)00025-3 [13] KARACA F, BOLAT E. Coprocessing of a Turkish lignite with a cellulosic waste material 1[J]. Fuel Process Technol, 2000, 64(1/3):47-55. doi: 10.1016-S0378-3820(00)00076-X/ [14] KARACA F, BOLAT E. Coprocessing of a Turkish lignite with a cellulosic waste material 2[J]. Fuel Process Technol, 2002, 75(2):109-116. doi: 10.1016/S0378-3820(01)00252-1 [15] LALVANI SB, MUCHMORE CB, KOROPCHAK J, ABASH B, CHIVATE P, CHAVEZT C. Lignin-augmented coal depolymerization under mild reaction conditions[J]. Energy Fuels, 1991, 5(2):347-352. doi: 10.1021-ef00026a021/ [16] GUO Z X, BAI Z Q, BAI J, WANG Z Q, LI W. Co-liquefaction of lignite and sawdust under syngas[J]. Fuel Process Technol, 2011, 92(1):119-125. doi: 10.1016/j.fuproc.2010.09.014 [17] CHEN C, GAO J S, YAN Y J. Observation of the type of hydrogen bonds in coal by FTIR[J]. Energy Fuels, 1998, 12(3):446-449. doi: 10.1021/ef970100z [18] CAI M F, SMART R B. Comparison of seven west Virginia coals with their N-methyl-2-pyrrolidinone-soluble extracts and residues. 1. Diffuse reflectance infrared Fourier transform spectroscopy[J]. Energy Fuels, 1994, 8(2):369-374. doi: 10.1021/ef00044a012 [19] DYRKACZ R A, BLOOMQUIST C A A. On the use of infrared spectroscopy to determine hydroxyl content and reactivity of O-acetylated and O-alkylated coals[J]. Energy Fuels, 1999, 13(1):40-52. doi: 10.1021/ef980062z [20] BENKHEDDA Z, LANDAIS P, KISTER J, DEREPPE J M, MONTHIOUX M. Spectroscopic analyses of aromatic hydrocarbons extracted from naturally and artificially matured coals[J]. Energy Fuels, 1992, 6(2):166-172. doi: 10.1021/ef00032a008 [21] WANG Z C, WEI C, SHUI H F, REN S B, PAN C X, WANG Z S, LI H P, LEI Z P. Synchronous fluorimetric characterization of heavy intermediates of coal direct liquefaction[J]. Fuel, 2012, 98(8):67-72. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=4c959ed19f605f17273018df43412501 [22] SHUI H F, CHEN Z X, WANG Z C, ZHANG D X. Kinetics of Shenhua coal liquefication catalyzed by SO42-/ZrO2 solid acid[J]. Fuel, 2010, 89(1):67-72. [23] KOYANO K, TAKANOHASHI T, SAITO I. Catalytic hydrogenation of HyperCoal (ashless coal) and reusability of catalyst[J]. Energy Fuels, 2009, 23(7):3652-3657. doi: 10.1021/ef900135r -





 下载:
下载: