Effect of Ce ion on adsorption and diffusion behavior of benzene in Y zeolite
-
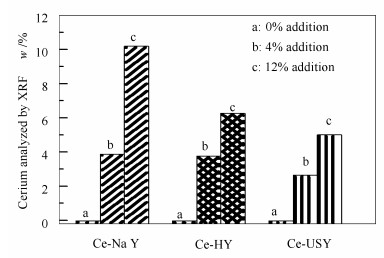
摘要: 采用液相离子交换法制备了不同稀土含量的Y型分子筛(HY、USY和NaY),研究了稀土铈(Ce)阳离子在Y型分子筛上吸附-脱附烃类分子(苯)过程中的作用机理与影响。通过X射线荧光光谱仪(XRF)、智能质量分析仪(IGA)、脱附指数的计算和巨正则蒙特卡罗模拟计算等多种表征计算方法,对引入稀土物种后,Y型分子筛对苯的饱和吸附量、吸附作用力、脱附热力学参数、苯在Y分子筛上的吸附势能分布及扩散行为等方面进行了研究。结果表明,Ce离子对苯在Y分子筛上脱附活化能的降低、吸附作用力的减弱以及吸附态由团聚态向分散态转变等方面具有显著影响,该作用构成了CeY分子筛催化剂在流化催化裂化(FCC)过程中能够优化轻质产品选择性的重要因素。Abstract: The liquid ion exchange method was employed to prepare cerium (Ce) cation modified Y zeolite (CeY) with various amount of cerium using different types of Y zeolite (HY, USY and NaY) and characterized by X-ray fluorescence spectrometry (XRF), intelligent gravimetric analyzer (IGA) and a molecular simulation technology (Grand Canonical Monte Carlo simulation, GCMC). A novel calculation method of desorption index (DI) has also been proposed to study the influence of cerium cations on the processes of adsorption-desorption of hydrocarbon molecule (benzene) on the CeY zeolites. The saturated adsorption capacity of benzene, adsorption interaction, desorption thermodynamic parameters, potential distribution curves and diffusion processes of benzene in CeY zeolites were analyzed. The results indicate that Ce ion can reduce the desorption activation energy, weaken the adsorption interaction force between benzene and Y zeolites, and modulate the adsorbed state of benzene molecules from agglomerate state to dispersed state, which are main factors to optimize the product selectivity of light oil components in the fluid catalytic cracking (FCC) process with CeY zeolite catalyst.
-
Key words:
- catalysis /
- Y-type zeolite /
- surface /
- sorbents /
- product selectivity /
- Monte Carlo simulation
-
表 1 Langmuir方程拟合C6H6在CeY分子筛中的吸附等温线数据
Table 1 Adsorption isotherm parameters of C6H6 in CeY zeolite fitted with Langmuir equations
Sample Qm K R2 NaY 255.20 0.12 0.984 13.3%CeY 254.16 0.15 0.973 42.3%CeY 245.07 0.12 0.972 71.3%CeY 226.80 0.10 0.980 99.7%CeY 206.02 0.07 0.975 表 2 苯与NaY和不同CeY分子筛最可几相互作用的能量(303 K)
Table 2 Interaction energies of benzene with NaY and CeY zeolites (303 K)
Energy E/(kJ·mol-1) NaY 13.3%CeY 42.3%CeY 71.3%CeY 99.7%CeY -87.99 -71.55 -69.16 -88.24 -108.28 -106.15 -98.87 -95.14 -103.85 -137.32 -125.27 -127.91 -134.52 -
[1] GABRIELA D L P, SEDRAN U. Conversion of methylcyclopentane on rare earth exchanged Y zeolite FCC catalysts[J]. Appl Catal A:Gen, 1996, 144(1/2):147-158. https://www.researchgate.net/publication/244106275_Conversion_of_methylcyclopentane_on_rare_earth_exchanged_Y_zeolite_FCC_catalysts [2] GABRIELA D L P, EDUARDO F S, FATIMA M, Zanon Z, VERA L D C. Influence of different rare earth ions on hydrogen transfer over Y zeolite[J]. Appl Catal A:Gen, 2000, 197(1):41-46. doi: 10.1016/S0926-860X(99)00531-1 [3] LIU X M, LIU S, LIU Y X. A potential substitute for CeY zeolite used in fluid catalytic cracking process[J]. Microporous Mesoporous Mater, 2016, 226:162-168. doi: 10.1016/j.micromeso.2015.12.046 [4] CERQUEIRA H S, CAEIRO G, COSTA L, RAMOA RIBEIRO F J. Deactivation of FCC catalysts[J]. J Mol Catal A:Chem, 2008, 292:1-13. doi: 10.1016/j.molcata.2008.06.014 [5] GUZMAN A, ZUAZO I, FELLER A, OLINDO R, SIEVERS C, LERCHER J A. On the formation of the acid sites in lanthanum exchanged X zeolites used for isobutane/cis-2-butene alkylation[J]. Microporous Mesoporous Mater, 2005, 83:309-318. doi: 10.1016/j.micromeso.2005.04.024 [6] ARBUZNIKOV A, VASILYEV V, GOURSOT A. Relationships between the structure of a zeolite and its adsorption properties[J].Surf Sci, 1998, 397:395-405. doi: 10.1016/S0039-6028(97)00760-7 [7] SONG L J, SUN Z L, BAN H Y, DAI M, REES L V C. Studies of unusual adsorption and diffusion behaviour of benzene in silicalite-1[J]. Chem Chem Phys, 2004, 6:4722-4731. doi: 10.1039/b406051b [8] BLIGAARD T, NÖRSKOV J K, DAHL S, MATTHIESEN J, CHRISTENSEN C H, SEHESTED J. The Brönsted-Evans-Polanyi relation and the volcano curve in heterogeneous catalysis[J]. J Catal, 2004, 224(1):206-217. doi: 10.1016/j.jcat.2004.02.034 [9] BEZVERKHYY I, RYZHIKOV A, GADACZ G, BELLAT J P. Kinetics of thiophene reactive adsorption on Ni/SiO2 and Ni/ZnO[J]. Catal Today, 2008, 130(1):199-205. doi: 10.1016/j.cattod.2007.06.038 [10] LEE E F T, REES L V C.Calcination of cerium (Ⅲ) exchanged Y zeolite[J]. Zeolites, 1987, 7(5):446-450. doi: 10.1016/0144-2449(87)90013-3 [11] LOPES J M, RIBEIRO F R.Effect of rare-earth nature on the basic properties of zeolite NaX containing occluded rare-earth species[J]. J Mol Catal A:Chem, 2002, 179:185-191. doi: 10.1016/S1381-1169(01)00324-7 [12] CERQUEIRA H S, CAEIRO G, COSTA L, RIBEIRO F R. Deactivation of FCC catalysts[J]. J Mol Catal A:Chem, 2008, 292:1-13. doi: 10.1016/j.molcata.2008.06.014 [13] SHU Y, TRAVERT A, SCHILLER R, ZIEBARTH M, WORMSBECHER R, CHENG C W. Effect of ionic radius of rare earth on USY zeolite in fluid catalytic cracking:Fundamentals and commercial application[J]. Top Catal, 2015, 58:334-342. doi: 10.1007/s11244-015-0374-0 [14] DU X H, GAO X H, ZHANG H T, LI X L, LIU P S. Effect of cation location on the hydrothermal stability of rare earth-exchanged Y zeolites[J]. Catal Commun, 2013, 35:17-22. doi: 10.1016/j.catcom.2013.02.010 [15] BOITON A P. The nature of rare-earth exchanged Y zeolites[J]. J Catal, 1971, 22(1):9-15. doi: 10.1016/0021-9517(71)90259-4 [16] SCHERZER J, RITTER R E. Ion-exchanged ultrastable Y zeolites 3 gas oil cracking over rare earth-exchanged ultrastable Y zeolites[J]. Ind Eng Chem Prod Res Dev, 1978, 17(3):219-223. doi: 10.1021/i360067a008 [17] 于善青, 田辉平, 代振宇, 龙军. La或Ce增强Y型分子筛结构稳定性的机制[J].催化学报, 2010, 31(10):1263-1270. http://www.cnki.com.cn/Article/CJFDTOTAL-CHUA201010015.htmYU Shan-qin, TIAN Hui-ping, DAI Zhen-yu, LONG Jun. Mechanism of the influence of lanthanum and cerium on the stability of Y zeolite[J]. Chin J Catal, 2010, 31(10):1263-1270. http://www.cnki.com.cn/Article/CJFDTOTAL-CHUA201010015.htm [18] SHU Y Y, TRAVERT A, SCHILLER R, MICHAEL Z, RICHARD W, WU C C. Effect of ionic radius of rare earth on USY zeolite in fluid catalytic cracking:Fundamentals and commercial application[J]. Top Catal, 2015, 58:334-342. doi: 10.1007/s11244-015-0374-0 [19] 卢天, 陈飞武.原子电荷计算方法的对比[J].物理化学学报, 2012, 28(1):1-18. http://www.cnki.com.cn/Article/CJFDTOTAL-WLHX201201002.htmLU Tian, CHEN Fei-wu.Comparison of computational methods for atomic charges[J]. Acta Phys-Chim Sin, 2012, 28(1):1-18. http://www.cnki.com.cn/Article/CJFDTOTAL-WLHX201201002.htm [20] 张乐, 秦玉才, 汲德强, 杨野, 贾未鸣, 宋丽娟.稀土铈离子迁移对Y分子筛活性中心的影响[J].稀土, 2016, 37(4):16-22.ZHANG Le, QIN Yu-cai, JI De-qiang, YANG Ye, JIA Wei-ming, SONG Li-juan. The effects of cerium ions migration on active sites of y zeolite[J]. Chin Rare Earth, 2016, 37(4):16-22. [21] LEE C K, ASHTEKAR S, GLADDEN L F, BARRIE P J. Adsorption and desorption kinetics of hydrocarbons in FCC catalysts studied using a tapered element oscillating microbalance (TEOM). Part 1:Experimental measurements[J]. J Chem Eng Sci, 2004, 59:1131-1138. doi: 10.1016/j.ces.2004.01.005 [22] BARRIE P J, LEE C K, GLADDEN L F. Adsorption and desorption kinetics of hydrocarbons in FCC catalysts studied using a tapered element oscillating microbalance (TEOM). Part 2:Numerical simulations[J].Chem Eng Sci, 2004, 59:1139-1151. doi: 10.1016/j.ces.2004.01.008 [23] 段林海.沸石分子筛的吸附扩散及应用[D].兰州:兰州大学, 2006.DUAN Lin-hai. Adsorption, diffusion on zeolite and it's application[D]. Lanzhou:Lanzhou University, 2006. [24] 刘道胜, 韩春玉, 段林海, 宋丽娟, 孙兆林.最小二乘法计算苯、噻吩和正辛烷在NaY上程序升温脱附活化能[J].物理化学学报, 2009, 25(3):470-476. http://www.cnki.com.cn/Article/CJFDTOTAL-WLHX200903014.htmLIU Dao-sheng, HAN Chun-yu, DUAN Lin-hai, SONG Li-juan, SUN Zhao-lin. Activation energy of temperature programmed desorption calculated using least-squares method for benzene, thiophene and octane on NaY[J]. Acta Phys-Chim Sin, 2009, 25(3):470-476. http://www.cnki.com.cn/Article/CJFDTOTAL-WLHX200903014.htm [25] BROIDO A J. Sensitive graphical method of treating thermogravimetric analysis data[J]. Polym Sci Part B:Polym Phys, 1969, 7(10):1761-1773. doi: 10.1002/pol.1969.160071012 [26] FALCONER J L, MADIX R J. Desorption rate isotherms in flash desorption analysis[J]. J Catal, 1977, 48(1/3):262-268. https://www.researchgate.net/publication/256213834_Desorption_rate_isotherms_in_flash_desorption_analysis [27] KISSINGER H E. Reaction kinetics in differential thermal analysis[J]. Anal Chem, 1957, 29(11):1703-1706. doi: 10.1021/ac60131a045 [28] YANG R T, STEINBERG M. Reaction kinetics and differential thermal analysis[J]. J Phys Chem, 1976, 80(9):965-968. doi: 10.1021/j100550a009 [29] 孙书红, 庞新梅, 郑淑琴, 张忠东.稀土超稳Y型分子筛催化裂化催化剂的研究[J].石油炼制与化工, 2001, 32(6):25-28. http://www.cnki.com.cn/Article/CJFDTOTAL-SYLH200106009.htmSUN Shu-hong, PANG Xin-mei, ZHENG Shu-qin, ZHANG Zhong-dong. Preparation of FCC catalyst containing REUSY[J]. Chin Pet Proc Pet Technol, 2001, 32(6):25-28. http://www.cnki.com.cn/Article/CJFDTOTAL-SYLH200106009.htm [30] SOUSA-AGUIAR E F, TRIGUEIRO F E, ZOTIN F M Z.The role of rare earth elements in zeolites and cracking catalysts[J]. Catal Today, 2013, 218-219:115-122. doi: 10.1016/j.cattod.2013.06.021 [31] 张乐, 高雄厚, 张艳惠, 苏怡, 张爱萍.钠含量对超稳Y分子筛物化性能的综合影响[J].人工晶体学报, 2014, 43(2):454-464. http://www.cnki.com.cn/Article/CJFDTOTAL-RGJT201402042.htmZHANG Le, GAO Xiong-hou, ZHANG Yan-hui. SU Yi, ZHANG Ai-ping. Effects of sodium content on physicochemical properties of USY zeolite[J]. J Synthetic Crystals, 2014, 43(2):454-464. http://www.cnki.com.cn/Article/CJFDTOTAL-RGJT201402042.htm [32] SANDOVAL-DÍAZ L E, MARTÍNEZ-GIL J M, TRUJILLO C A.The combined effect of sodium and vanadium contamination upon the catalytic performance of USY zeolite in the cracking of n-butane:Evidence of path-dependent behavior in Constable-Cremer plots[J]. J Catal, 2012, 294:89-98. doi: 10.1016/j.jcat.2012.07.009 -





 下载:
下载: