Experimental study on benzene removal of fuel gas in a packed-bed dielectric barrier discharge reactor
-
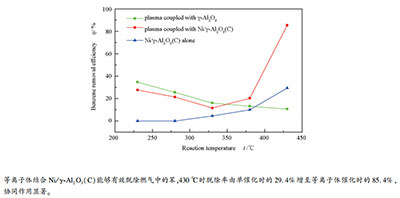
摘要: 以生物质气化焦油典型组分——苯作为模型物,采用填充床介质阻挡放电(DBD)对气化燃气氛围中的苯进行脱除。考察了燃气组成、填充物种类、反应温度及催化剂还原方式对苯脱除的影响。结果表明,反应温度200 ℃时,空气气化燃气与水蒸气气化燃气氛围内的苯脱除率比较接近,但燃气中存在少量O2会导致脱除率明显下降。并且,提高放电能量密度,使用高介电常数、高比表面积及孔容积的填充物能提高苯脱除率。采用传统还原和等离子体还原两种方式分别制得Ni/γ-Al2O3(C)、Ni/γ-Al2O3(P)催化剂,以Ni/γ-Al2O3(C)为DBD填充物,反应温度在230-330 ℃时,苯脱除率随温度升高而下降,330 ℃时达到最低脱除率11.6%;温度高于330 ℃,苯脱除率随温度急剧上升且在430 ℃达到最大值85.4%。等离子体还原可制得大比表面积及高分散性的Ni/γ-Al2O3(P),其苯脱除率随温度变化的趋势与Ni/γ-Al2O3(C)一致,但在430 ℃时达到更高的脱除率90.0%。苯脱除过程中燃气的甲烷化可提高出口燃气中CH4浓度,但燃气的热值略有下降。Abstract: A packed-bed dielectric barrier discharge (DBD) reactor was developed to investigate the removal of biomass tar in fuel gas atmosphere, and benzene was used as the tar surrogate. The effects of fuel gas composition, packing materials, reaction temperature and reduction methods of catalysts on the removal efficiency of benzene were investigated. The results indicate that the benzene removal efficiency of air-gasification fuel gas is close to that of steam-gasification fuel gas at low temperatures, but the presence of O2 in the fuel gas leads to a large drop in the removal efficiency. In addition, the enhancement of the plasma discharge power and the use of packing materials with higher permittivity, specific surface area and pore volume can improve the benzene removal efficiency. For the plasma-catalytic process, the combination of DBD plasma and Ni/γ-Al2O3 (C) shows a significant benzene removal potential. The benzene removal efficiency decreases with temperature from 230-330℃, reaching a minimum value of 11.6%, and then notably increases to 85.4% at 430℃. Furthermore, the combination of plasma and Ni/γ-Al2O3 (P), which is reduced by plasma under H2 atmosphere, has a similar tendency of benzene removal behavior within the temperature range of 230-430℃, reaching a maximum removal efficiency of 90.0% at 430℃ due to higher specific surface area and nickel dispersion of Ni/γ-Al2O3 (P). Moreover, the increased CH4 concentration induced by the methanation of the fuel gas and the slightly decreased heating value of the fuel gas are obtained in the plasma-catalytic process.
-
Key words:
- biomass gasification /
- fuel gas /
- tar /
- benzene /
- non-thermal plasma /
- catalysis
-
图 8 不同反应温度下反应器出口处燃气各组分的浓度
Figure 8 Concentrations of H2, CO, CO2 and CH4 at the outlet as a function of reaction temperature in plasma-catalytic process (a) and catalytic alone process (b)
reaction conditions: specific energy input=~350 J/L, packing material: Ni/γ-Al2O3(C), carrier gas: air-gasification fuel gas 1
表 1 不同填充物的比表面积和孔结构
Table 1 Specific surface area and pore structure of packing materials
Packing material Specific surface area A/(m2·g-1) Pore volume v/(cm3·g-1) Average diameter d/nm Glass pellets < 1 - - γ-Al2O3 94 0.343 7.3 Ni/γ-Al2O3(P) 84 0.311 7.4 Ni/γ-Al2O3(C) 79 0.312 7.8 表 2 不同类型的燃气组成
Table 2 Gas components of different gasification fuel gases
Gas mixture Component φ/% H2 CO CO2 CH4 N2 O2 Air-gasification fuel gas 1 15 18 12 1.5 53.5 - Air-gasification fuel gas 2 10 22 15 3 50 - Steam-gasification fuel gas 40 25 25 8 2 - Oxygen-containing fuel gas 10 22 15 3 48 2 表 3 不同等离子体反应器内焦油脱除性能对比
Table 3 Performance comparison of tar removal by different plasma reactors
Process Target Carrier
gasQ a/
(L·min-1)t/℃ Tar content/
(g·m-3)η/% E/
(g·(kW·h)-1)SEI/
(kJ·L-1)Ref. Microwave
plasmaC6H6 N2+H2O 20.0 n.a.b 15.0 90.0 8.8 6.0 [31] AC gliding arc
dischargeC6H6 N2+H2O 16.7 n.a. 3.7 82.6 20.9 0.61 [32] AC gliding arc
dischargeC7H8 N2+H2O 3.8 n.a. 23.5 35.8 46.3 0.68 [33] Rotating gliding arc
dischargeC7H8 N2 10.0 n.a. 14.0 83.0 16.6 2.5 [10] DBD with
Ni/Al2O3C7H8 N2+H2O 0.15 < 200 17.7 52.0 2.6 14.0 [12] DBD with
Ni/Al2O3C7H8 N2+H2O 0.15 300 180.0 96.0 25.0 25.7 [13] DBD with
Ni/Al2O3C6H6 Fuel gas 1.0 430 1.9 85.4 16.7 0.35 this
worka: Q represents the total flow rate; b: n.a. = not available 表 4 不同温度下等离子体结合催化剂反应器出口处的燃气低位热值
Table 4 LHV of fuel gas at the outlet as a function of reaction temperature in plasma-catalytic process
Reaction temperature QLHV /(MJ·m-3) Growth rate /% Air-gasification fuel gas 1 4.43 - 230 ℃ 4.35 -1.7 280 ℃ 4.36 -1.4 330 ℃ 4.19 -5.3 380 ℃ 4.18 -5.6 430 ℃ 4.24 -4.4 -
[1] KUMAR A, DEMIREL Y, JONES D D, HANNA M A. Optimization and economic evaluation of industrial gas production and combined heat and power generation from gasification of corn stover and distillers grains[J]. Bioresour Technol, 2010, 101(10):3696-3701. doi: 10.1016/j.biortech.2009.12.103 [2] LEIBBRANDT N H, ABOYADE A O, KNOETZE J H, GÖRGENS J F. Process efficiency of biofuel production via gasification and Fischer-Tropsch synthesis[J]. Fuel, 2013, 109(7):484-492. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=dca5c0956cc49bfe32bb7b133261c7e2 [3] LI C S, SUZUKI K. Tar property, analysis, reforming mechanism and model for biomass gasification-An overview[J]. Renewable Sustainable Energy Rev, 2009, 13(3):594-604. doi: 10.1016/j.rser.2008.01.009 [4] ANIS S, ZAINAL Z A. Tar reduction in biomass producer gas via mechanical, catalytic and thermal methods:A review[J]. Renewable Sustainable Energy Rev, 2011, 15(5):2355-2377. doi: 10.1016/j.rser.2011.02.018 [5] SHEN Y, YOSHIKAWA K. Recent progresses in catalytic tar elimination during biomass gasification or pyrolysis:A review[J]. Renewable Sustainable Energy Rev, 2013, 21:371-392. doi: 10.1016/j.rser.2012.12.062 [6] TATAROVA E, BUNDALESKA N, SARRETTE J P, FERREIRA C M. Plasmas for environmental issues:from hydrogen production to 2D materials assembly[J]. Plasma Sources Sci T, 2014, 23(6):063002. doi: 10.1088/0963-0252/23/6/063002 [7] OBRADOVIĆB M, SRETENOVIĆ G B, KURAICA M M. A dual-use of DBD plasma for simultaneous NOx and SO2 removal from coal-combustion flue gas[J]. J Hazard Mater, 2011, 185(2/3):1280-1286. http://www.sciencedirect.com/science/article/pii/S0304389410013312 [8] CHUNG W C, PAN K L, LEE H M, CHANG M B. Dry reforming of methane with dielectric barrier discharge and ferroelectric packed-bed reactors[J]. Energy Fuels, 2016, 28(12):7621-7631. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=1a5fe05f8963120c646c360bc04b6015 [9] TU X, WHITEHEAD J C. Plasma-catalytic dry reforming of methane in an atmospheric dielectric barrier discharge:Understanding the synergistic effect at low temperature[J]. Appl Catal B:Environ, 2012, 125(Supplement C):439-448. http://www.sciencedirect.com/science/article/pii/S0926337312002597 [10] ZHU F, LI X, ZHANG H, WU A, YAN J, NI M, ZHANG H, BUEKENS A. Destruction of toluene by rotating gliding arc discharge[J]. Fuel, 2016, 176:78-85. doi: 10.1016/j.fuel.2016.02.065 [11] NAIR S A, PEMEN A J M, YAN K, VAN GOMPEL F M, VAN LEUKEN H E M, VAN HEESCH E J M, PTASINSKI K J, DRINKENBURG A A H. Tar removal from biomass-derived fuel gas by pulsed corona discharges[J]. Fuel Process Technol, 2003, 84(1/3):161-173. [12] LIU S Y, MEI D H, NAHIL M A, GADKARI S, GU S, WILLIAMS P T, TU X. Hybrid plasma-catalytic steam reforming of toluene as a biomass tar model compound over Ni/Al2O3 catalysts[J]. Fuel Process Technol, 2017, 166:269-275. doi: 10.1016/j.fuproc.2017.06.001 [13] LIU L, WANG Q, AHMAD S, YANG X, JI M, SUN Y. Steam reforming of toluene as model biomass tar to H2-rich syngas in a DBD plasma-catalytic system[J]. J Energy Inst, 2017, 91(6):927-939. http://www.sciencedirect.com/science/article/pii/S1743967117305755 [14] ZORAN F, JOHN J C. Microdischarge behaviour in the silent discharge of nitrogen-oxygen and water-air mixtures[J]. J Phys D Appl Phys, 1997, 30(5):817-825. doi: 10.1088/0022-3727/30/5/015 [15] GOUJARD V, TATIBOUËT J M, BATIOT-DUPEYRAT C. Carbon dioxide reforming of methane using a dielectric barrier discharge reactor:Effect of helium dilution and kinetic model[J]. Plasma Chem Plasma Process, 2011, 31(2):315-325. doi: 10.1007/s11090-010-9283-y [16] COLL R, SALVADÍ J, FARRIOL X, MONTANÉ D. Steam reforming model compounds of biomass gasification tars:conversion at different operating conditions and tendency towards coke formation[J]. Fuel Process Technol, 2001, 74(1):19-31. http://www.sciencedirect.com/science/article/pii/S0378382001002144 [17] 董新新, 金保昇, 王妍艳, 牛淼淼. Ni/γ-Al2O3甲烷化催化剂提高生物质气化燃气低位热值的实验[J].东南大学学报(英文版), 2017, 33(4):448-456. doi: 10.3969/j.issn.1003-7985.2017.04.010DONG Xin-xin, JIN Bao-sheng, WANG Yan-yan, NIU Miao-miao. Experiments on Ni/γ-Al2O3 catalyst for improving lower heating value of biomass gasification fuel gas via methanation[J]. J Southeast Univ, 2017, 33(4):448-456. doi: 10.3969/j.issn.1003-7985.2017.04.010 [18] PATCAS F, HÖNICKE D. Effect of alkali doping on catalytic properties of alumina-supported nickel oxide in the selective oxidehydrogenation of cyclohexane[J]. Catal Commun, 2005, 6(1):23-27. doi: 10.1016/j.catcom.2004.10.005 [19] ZHANG J, XU H, JIN X, GE Q, LI W. Characterizations and activities of the nano-sized Ni/Al2O3 and Ni/La-Al2O3 catalysts for NH3 decomposition[J]. Appl Catal A:Gen, 2005, 290(1):87-96. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=64fe73601ba4d6df1db80ffb48907620 [20] NEYTS E C, BOGAERTS A. Understanding plasma catalysis through modelling and simulation-a review[J]. J Phys D Appl Phys, 2014, 47(22):224010. doi: 10.1088/0022-3727/47/22/224010 [21] GIL J, CORELLA J, AZNAR M P, CABALLERO M A. Biomass gasification in atmospheric and bubbling fluidized bed:Effect of the type of gasifying agent on the product distribution[J]. Biomass Bioenergy, 1999, 17(5):389-403. doi: 10.1016/S0961-9534(99)00055-0 [22] NAIR S A, PEMEN A J M, YAN K, VAN HEESCH E J M, PTASINSKI K J, DRINKENBURG A A H. Chemical processes in tar removal from biomass derived fuel gas by pulsed corona discharges[J]. Plasma Chem Plasma Process, 2003, 23(4):665-680. doi: 10.1023/A:1025510402107 [23] NAIR S A. Corona plasma for tar removal[J]. Eindhoven University of Technology, Eindhoven, The Netherlands, 2004. [24] BITYURIN V A, FILIMONOVA E A, NAIDIS G V. Simulation of naphthalene conversion in biogas initiated by pulsed corona discharges[J]. Ieee Trans Plasma Sci, 2009, 37(6):911-919. doi: 10.1109/TPS.2009.2019756 [25] ABDELAZIZ A A, SETO T, ABDEL-SALAM M, OTANI Y. Influence of nitrogen excited species on the destruction of naphthalene in nitrogen and air using surface dielectric barrier discharge[J]. J Hazard Mater, 2013, 246/247:26-33. doi: 10.1016/j.jhazmat.2012.12.005 [26] 陈春雨, 刘彤, 王卉, 于琴琴, 范杰, 肖丽萍, 郑小明.低温等离子体与MnOx/γ-Al2O3协同催化降解正己醛[J].催化学报, 2012, 33(6):941-951. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=cuihuaxb201206005CHENG Chun-yu, LIU Tong, WANG Hui, YU Qin-qin, FAN Jie, XIAO Li-ping, ZHENG Xiao ming. Removal of hexanal by Non-thermal plasam and MnOx/γ-Al2O3 combination[J]. Chin J Catal, 2012, 33(6):941-951. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=cuihuaxb201206005 [27] JO S, KIM T, LEE D H, KANG W S, SONG Y H. Effect of the electric conductivity of a catalyst on methane activation in a dielectric barrier discharge reactor[J]. Plasma Chem Plasma P, 2014, 34(1):175-186. doi: 10.1007/s11090-013-9505-1 [28] BLACKBEARD T, DEMIDYUK V, HILL S L, WHITEHEAD J C. The effect of temperature on the plasma-catalytic destruction of propane and propene:A comparison with thermal catalysis[J]. Plasma Chem Plasma P, 2009, 29(6):411-419. doi: 10.1007/s11090-009-9189-8 [29] WANG Q, YAN BH, JIN Y, CHENG Y. Dry reforming of methane in a dielectric barrier discharge reactor with Ni/Al2O3 catalyst:Interaction of catalyst and plasma[J]. Energy Fuels, 2009, 23(8):4196-4201. doi: 10.1021/ef900286j [30] HARLING A M, KIM H-H, FUTAMURA S, WHITEHEAD J C. Temperature dependence of plasma-catalysis using a nonthermal, atmospheric pressure packed bed, the destruction of benzene and toluene[J]. J Phys Chem C, 2007, 111(13):5090-5095. doi: 10.1021/jp067821w [31] JAMRÍZ P, KORDYLEWSKI W, WNUKOWSKI M. Microwave plasma application in decomposition and steam reforming of model tar compounds[J]. Fuel Process Technol, 2018, 169:1-14. doi: 10.1016/j.fuproc.2017.09.009 [32] CHUN Y N, KIM S C, YOSHIKAWA K. Decomposition of benzene as a surrogate tar in a gliding Arc plasma[J]. Environ Prog Sustainable Energy, 2013, 32(3):837-845. doi: 10.1002/ep.11663 [33] LIU S, MEI D, WANG L, TU X. Steam reforming of toluene as biomass tar model compound in a gliding arc discharge reactor[J]. Chem Eng J, 2017, 307:793-802. doi: 10.1016/j.cej.2016.08.005 [34] SHANG S, LIU G, CHAI X, TAO X, LI X, BAI M, CHU M, DAI X, ZHAO Y, YIN Y. Research on Ni/γ-Al2O3 catalyst for CO2 reforming of CH4 prepared by atmospheric pressure glow discharge plasma jet[J]. Catal Today, 2009, 148(3):268-274. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=c4955582879a8cf2e8d96e26eea91cbc [35] 张旭, 孙文晶, 储伟.等离子体技术对CO2甲烷化用Ni/SiO2催化剂的改性作用[J].燃料化学学报, 2013, 41(1):96-101. doi: 10.3969/j.issn.0253-2409.2013.01.016ZHANG Xu, SUN Wen-jing, CHU Wei. Effect of glow discharge plasma treatment on the performance of Ni/SiO2 catalyst in CO2 methanation[J]. J Fuel Chem Technol, 2013, 41(1):96-101. doi: 10.3969/j.issn.0253-2409.2013.01.016 [36] 柴晓燕, 尚书勇, 刘改焕, 陶旭梅, 李祥, 白玫瑰, 戴晓雁, 印永祥.常压高频冷等离子体炬制备的CH4/CO2重整用Ni/γ-Al2O3催化剂的表征[J].催化学报, 2010, 31(3):353-359. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=cuihuaxb201003019CHAI Xiao-yan, SHANG Shu-yong, LIU Gai-huan, TAO Xu-mei, LI Xiang, BAI Mei-gui, DAI Xiao-yan, YIN Yong-xiang. Characterization of Ni/γ-Al2O3 catalyst prepared by atmospheric high frequency cold plasma jet for CO2 reforming of CH4[J]. Chin J Catal 2010, 31(3):353-359. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=cuihuaxb201003019 [37] HUA W, JIN L, HE X, LIU J, HU H. Preparation of Ni/MgO catalyst for CO2 reforming of methane by dielectric-barrier discharge plasma[J]. Catal Commun, 2010, 11(11):968-972. doi: 10.1016/j.catcom.2010.04.007 [38] 黄秋实, 兰俐颖, 王安杰, 王瑶.二氧化碳甲烷化反应中等离子体与Ni/ZSM-5催化剂的协同作用[J].石油化工, 2017, 46(11):1355-1360. doi: 10.3969/j.issn.1000-8144.2017.11.002HUANG Qiu-shi, LAN Li-ying, WANG An-jie, WANG Yao. Synergy of non-thermal plasma and Ni/ZSM-5 in CO2 methanation[J]. Petrochem Technol, 2017, 46(11):1355-1360. doi: 10.3969/j.issn.1000-8144.2017.11.002 [39] CHOUDHURY M B I, AHMED S, SHALABI M A, INUI T. Preferential methanation of CO in a syngas involving CO2 at lower temperature range[J]. Appl Catal A:Gen, 2006, 314(1):47-53. doi: 10.1016/j.apcata.2006.08.008 [40] KOPYSCINSKI J, SCHILDHAUER T J, BIOLLAZ S M A. Production of synthetic natural gas (SNG) from coal and dry biomass-A technology review from 1950 to 2009[J]. Fuel, 2010, 89(8):1763-1783. doi: 10.1016/j.fuel.2010.01.027 [41] 符世龙, 陈应泉, 杨海平.生物质合成气甲烷化催化剂研究进展[J].沈阳农业大学学报, 2017, 48(4):488-496. http://d.old.wanfangdata.com.cn/Periodical/synydxxb201704017FU Shi-long, CHEN Yin-quan, YANG Hai-ping. Research advances in methanation catalysts for bio-syngas[J]. J Shenyang Agr Univ, 2017, 48(4):488-496. http://d.old.wanfangdata.com.cn/Periodical/synydxxb201704017 [42] 武宏香, 赵增立, 王小波, 郑安庆, 李海滨, 何方.生物质气化制备合成天然气技术的研究进展[J].化工进展, 2013, 32(1):83-90+113. http://d.old.wanfangdata.com.cn/Periodical/hgjz201301013WU Hong-xiang, ZHAO Zeng-li, WANG Xiao-bo, ZHENG An-qing, LI Hai-bin, HE Fang. Technical development on synthetic natural gas production from biomass[J]. Chem Ind Eng Prog, 2013, 32(1):83-90+113. http://d.old.wanfangdata.com.cn/Periodical/hgjz201301013 -





 下载:
下载: