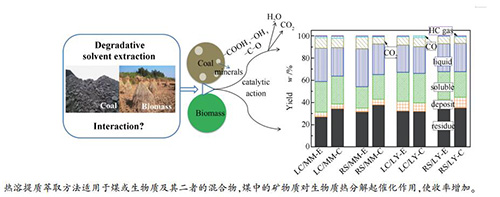
Interaction between low-rank coal and biomass during degradative solvent extraction
-
摘要: 作者前期工作中提出了一种针对生物质废弃物或低阶煤的热溶提质萃取方法,该方法可以在350℃下实现生物质或低阶煤的提质及多级分离,获得高附加值的萃取物。与生物质的热溶提质萃取比较,低阶煤的萃取物收率较低。本研究提出对生物质和低阶煤混合物进行热溶提质萃取,通过两者的物理化学相互作用,来提高其萃取收率。研究结果表明,对两者混合物热溶剂提质分离得到萃取物的产率及元素组成与理论计算结果略有不同,理论计算结果是假设生物质与煤之间无交互作用而得到的。煤中的矿物质对生物质热分解起催化作用,导致收率有所增加。煤、生物质及其混合物通过热溶剂提质分离得到的萃取物的元素组成、分子量分布、化学结构、热分解和热塑性非常相似。总体而言,低阶煤与生物质在热溶剂提质萃取过程的交互作用较小。但是,本研究证明了热溶提质萃取方法不仅适用于煤或生物质,也适用于它们的混合物。这意味着热溶剂提质萃取法可以在不改变任何工艺条件参数的情况下,以煤、生物质及其混合物作为原料。Abstract: A degradative solvent extraction at around 350℃ for low-rank coal or biomass wastes upgrading and fractionation was proposed in our previous work. The extraction yield of low-rank coal is relatively lower than that of biomass. In this work the blends of low-rank coal and biomass were treated by this method at 350℃ to investigate the interaction between them. The results showed that the yields and elemental compositions of the extracts obtained from the blends were slight different to the calculated results, which were calculated by assuming that there was no interaction between the coal and biomass. The slight promotion of yield was judged to be caused by the catalytic action of the minerals in the coal for thermal decomposition of biomass. It was worth to note that the elemental composition, molecular weight distribution, chemical structure, thermal decomposition behavior and thermoplastic behavior of the extracts obtained from low-rank coal, biomass and their blend, were rather similar to each other, independent of the properties of the raw feedstocks. Overall, the interaction between low-rank coal and biomass during the extraction was not significant. On the other hand, the proposed degradative solvent extraction method was fit not only by single low-rank coal and biomass but also by their blends to produce the product having similar physicochemical properties. This implied that an industrial system of degradative solvent extraction can use coal, biomass or their blends as feedstock at the same time without modification or adjustment.
-
Key words:
- low-rank coal /
- biomass /
- blends /
- degradative solvent extraction /
- upgrading
-
Table 1 Ultimate and proximate analyses of raw materials
Sample Ultimate analysis wdaf/% Proximate analysis w/% C H N O* Mad Vdb FCdb Adb Rice straw(RS) 49.8 7.0 1.0 42.2 8.5 73.0 11.9 15.1 Leucaena(LC) 49.3 6.6 1.4 42.7 11.2 82.1 16.6 1.3 Mae moh coal (MM) 66.4 3.9 1.9 27.8 21.3 50.2 24.0 25.8 Loy yang coal (LY) 66.7 4.7 0.9 27.7 42.5 51.5 47.0 1.5 *: calculated by difference Table 2 Ultimate and proximate analyses of products
Sample Ultimate analysis wdaf/% Proximate analysis wdb/% Atomic ratio C H N O* V FC A H/C O/C residuel 79.5 5.7 2.0 12.8 24.0 22.8 53.2 0.86 0.12 RS deposit 81.4 5.5 1.6 11.5 37.9 60.0 2.1 0.81 0.11 soluble 80.1 6.4 1.4 12.1 76.4 22.8 0.8 0.96 0.11 residuel 86.4 5.3 1.9 6.4 35.7 54.9 9.4 0.74 0.06 LC deposit 76.5 5.5 2.6 15.4 36.5 62.1 1.4 0.86 0.15 soluble 81.5 6.4 2.2 9.9 75.2 24.8 0.0 0.94 0.09 residuel 69.8 3.8 3.4 25.0 27.1 34.4 38.5 0.65 0.27 MM deposit 76.4 5.1 3.8 14.7 39.9 58.7 1.4 0.80 0.14 soluble 82.4 7.4 2.3 7.8 78.7 21.1 0.2 1.08 0.07 residuel 77.4 4.0 1.0 17.6 31.8 66.0 2.3 0.62 0.17 LY deposit 77.5 5.0 1.0 16.9 42.2 57.2 0.7 0.77 0.16 soluble 81.8 7.5 0.5 10.2 79.5 20.2 0.3 1.10 0.09 residuel 69.0 4.5 2.1 24.4 32.5 32.7 34.8 0.78 0.27 LC/MM deposit 75.6 5.3 2.3 16.9 38.8 60.5 0.7 0.84 0.17 soluble 80.1 6.7 1.3 11.9 76.9 23.1 0.0 1.0 0.11 residuel 64.9 4.6 1.9 28.7 16.7 39.3 44.0 0.85 0.33 RS/MM deposit 76.9 5.1 2.5 15.5 39.2 58.4 2.4 0.80 0.15 soluble 80.0 7.0 1.7 11.3 74.2 25.4 0.4 1.05 0.11 residuel 75.8 4.0 1.1 19.1 30.4 66.3 3.3 0.63 0.19 LC/LY deposit 74.3 4.9 1.2 19.7 41.9 58.1 0.0 0.79 0.20 soluble 80.0 6.7 0.7 12.6 73.1 26.9 0.0 1.01 0.12 residuel 73.0 4.1 1.4 21.6 27.1 54.6 18.3 0.67 0.22 RS/LY deposit 77.0 5.2 1.6 16.2 42.3 57.1 0.5 0.81 0.16 soluble 81.2 7.1 0.9 10.9 77.2 22.8 0.0 1.05 0.10 *: calculated by difference -
[1] WU F P, LU H, YAN J, WANG R Y, ZHAO Y P, WEI X Y. Differences in molecular composition of soluble organic species in two Chinese sub-bituminous coals with different reducibility[J]. J Fuel Chem Technol, 2018, 46(7):769-777. doi: 10.1016/S1872-5813(18)30033-1 [2] JING G, ZHENG D. Thermodynamic analysis of low-rank-coal-based oxygen-thermal acetylene manufacturing process system[J]. Ind Eng Chem Res, 2012, 51(41):13414-13422. doi: 10.1021/ie301986q [3] LI X, ZHU X Q, XIAO L, ASHIDA R, MIURA K, LUO G Q, YAO H. Degradative solvent extraction of demineralized and ion-exchanged low-rank coals[J]. J Fuel Chem Technol, 2014, 42(8):897-904. doi: 10.1016/S1872-5813(14)60038-4 [4] HASSAN K, RAJENDER G. Low-grade coals:A review of some prospective upgrading technologies[J]. Energy Fuels, 2009, 23(7):3392-3405. doi: 10.1021/ef801140t [5] AYHAN D. Biomass resource facilities and biomass conversion processing for fuels and chemicals[J]. Energy Convers Manage, 2001, 42(11):1357-1378. doi: 10.1016/S0196-8904(00)00137-0 [6] DAVID J B, EARL W E, JEFFRE M P. An overview of peat research, utilization, and environmental considerations[J]. Int J Coal Geol, 1987, 8(1):1-31. http://www.sciencedirect.com/science/article/pii/0166516287900206 [7] AKGUL O, ZAMBONI A, BEZZO F, SHAH N, PAPAGEORGIOU L G. Optimization-based approaches for bioethanol supply chains[J]. Ind Eng Chem Res, 2011, 50(9):4927-4938. doi: 10.1021/ie101392y [8] MIURA K, SHIMADA M, MAE K. Extraction of coal at 300 to 350℃ to produce precursors for chemicals[C]. 15th International Pittsburgh Coal Conference, Pittsburgh, 1998, 30-31. https://www.osti.gov/biblio/349224-extraction-coal-produce-precursors-chemicals [9] WANNAPEERA J, LI X, WORASUWANNARAK N, MIURA K, ASHIDA R. Production of high-grade carbonaceous materials and fuel having similar chemical and physical properties from various types of biomass by degradative solvent extraction[J]. Energy Fuels, 2012, 26:4521-4531. doi: 10.1021/ef3003153 [10] LI X, ASHIDA R, MIURA K. Preparation of high-grade carbonaceous materials having similar chemical and physical properties from various low-rank coals by degradative solvent extraction[J]. Energy Fuels, 2012, 26:6897-6904. doi: 10.1021/ef301364p [11] ZHU X Q, XUE Y, LI X, ZHANG Z, SUN W, ASHIDA R, MIURA K, YAO H. Mechanism study of degradative solvent extraction of biomass[J]. Fuel, 2016, 165:10-18. doi: 10.1016/j.fuel.2015.10.021 [12] LI X, ZHANG Z, ZHANG L, ZHU X Q, HU Z Z, QIAN W X, ASHIDA R, MIURA K, HU H Y, LUO G Q, YAO H. Degradative solvent extraction of low-rank coals by the mixture of low molecular weight extract and solvent as recycled solvent[J]. Fuel Process Technol, 2018, 173:48-55. doi: 10.1016/j.fuproc.2018.01.005 [13] ZHU X Q, TANG J, LI X, LAN W, XU K, FANG Y, ASHIDA R, MIURA K, LUO G Q, YAO H. Modelling and kinetic study of degradative solvent extraction of biomass wastes[J]. Energy Fuels, 2017, 31(5):5097-5103. doi: 10.1021/acs.energyfuels.6b03442 [14] OKUDA K, LI X, ASHIDA R, MIURA K. Carbon fiber preparation by low-molecular-weight extracts obtained from low-rank coal or biomass by degradative solvent extraction[C]. 19th Regional Symposium of Chemical Engineering, Bali Indonesia, 2012: 8-9. [15] MIURA K, MIYABAYASHI K, KAWANARI M, ASHIDA R. Enhancement of reduction rate of iron ore by utilizing low grade iron ore and brown coal derived carbonaceous materials[C]. The Iron and Steel Institute of Japan, 2011, 51: 6. [16] ZHU X Q, LI X, XIAO L, ZHANG X, TONG S, WU C, ASHIDA R, LIU W, MIURA K, YAO H. Novel carbon-rich additives preparation by degradative solvent extraction of biomass wastes for coke-making[J]. Bioresource Technol, 2016, 207:85-91. doi: 10.1016/j.biortech.2016.01.105 [17] LI X, PRIYANTO D E, ASHIDA R, MIURA K. Two-stage conversion of low-rank coal or biomass into liquid fuel under mild conditions[J]. Energy Fuels, 2015, 29:3127-3133. doi: 10.1021/ef502574b [18] LI X, ASHIDA R, MAKINO M, NISHIDA A, YAO H, MIURA K. Enhancement of gasification reactivity of low-rank coal through high-temperature solvent treatment[J]. Energy Fuels, 2014, 28:5690-5695. doi: 10.1021/ef501305s [19] LI J H, ZHOU Q X, MI H Y, LI X, LI H P. Preparation and capacitive properties of graphite-like porous carbon based on coal extracts[J]. J Inorg Mater, 2016, 1:39-46. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=wjclxb201601007 [20] LI X, ZHU X Q, OKUDA K, ZHANG Z, ASHIDA R, YAO H, MIURA K. Preparation of carbon fibers from low-molecular-weight compounds obtained from low-rank coal and biomass by solvent extraction[J]. New Carbon Mater, 2017, 32(1):41-47. doi: 10.1016/S1872-5805(17)60106-9 [21] ZHU X Q, TONG S, LI X, GAO Y X, XU Y, DACRES O D, ASHIDA R, MIURA K, LIU W Q, YAO H. Conversion of biomass into high-quality bio-oils by degradative solvent extraction combined with subsequent pyrolysis[J]. Energy Fuels, 2017, 31(4):3987-3994. doi: 10.1021/acs.energyfuels.6b03162 [22] ZHU X Q, ZHANG Z, ZHOU Q X, CAI T, QIAO E, LI X, YAO H. Upgrading and multistage separation of rice straw by degradative solvent extraction[J]. J Fuel Chem Technol, 2015, 43(4):422-428. doi: 10.1016/S1872-5813(15)30010-4 [23] LIU F J, WEI X Y, FAN M, ZONG Z M. Separation and structural characterization of the value-added chemicals from mild degradation of lignites:A review[J]. Appl Energy, 2016, 170:415-436. doi: 10.1016/j.apenergy.2016.02.131 [24] HU M, CHEN Z, WANG S, GUO D, MA C, ZHOU Y, CHEN J, LAGHARI M, FAZAL S, XIAO B, ZHANG B, MA S. Thermogravimetric kinetics of lignocellulosic biomass slow pyrolysis using distributed activation energy model, Fraser-Suzuki deconvolution, and iso-conversional method[J]. Energy Convers Manage, 2016, 118:1-11. doi: 10.1016/j.enconman.2016.03.058 [25] YANG H, YAN R, CHEN H, ZHENG C, LEE D H, LIANG D T. In-depth investigation of biomass pyrolysis based on three major components:Hemicellulose, cellulose and lignin[J]. Energy Fuels, 2006, 20:388-393. doi: 10.1021/ef0580117 [26] CHEN B, WEI X Y, ZONG Z M, YANG Z S, QING Y, LIU C. Difference in chemical composition of supercritical methanolysis products between two lignites[J]. Appl Energy, 2011, 88:4570-4576. doi: 10.1016/j.apenergy.2011.05.052 [27] LU H Y, WEI X Y, YU R, PENG Y L, QI X Z, QIE L M, WEI Q, LV J, ZONG Z M, ZHAO W, ZHAO Y P, NI Z H, WU L. Sequential thermal dissolution of huolinguole lignite in methanol and ethanol[J]. Energy Fuels, 2011, 25:2741-2745. doi: 10.1021/ef101734f [28] CARLSON T, TOMPSETT G, CONNER W, HUBER G. Aromatic production from catalytic fast pyrolysis of biomass-derived feedstocks[J]. Top Catal, 2009, 52:241-252. doi: 10.1007/s11244-008-9160-6 [29] MENG A, ZHOU H, QIN L, ZHANG Y, LI Q. Quantitative and kinetic TG-FTIR investigation on three kinds of biomass pyrolysis[J]. J Anal Appl Pyrolysis, 2013, 104:28-37. doi: 10.1016/j.jaap.2013.09.013 [30] LI X, OKUDA K, ASHIDA R, MIURA K, LUO G, YAO H. Carbon Fibers Preparation by Biomass or Low-Rank Coal Extracts Obtained by Degradative Solvent Extraction[C]. International Symposium on EcoTopia Science, Nagoya Japan, 2013. [31] TIAN C, LIU Z, ZHANG Y, LI B, CAO W, LU H, DUAN N, ZHANG L, ZHANG T. Hydrothermal liquefaction of harvested high-ash low-lipid algal biomass from Dianchi Lake:Effects of operational parameters and relations of products[J]. Bioresource Technol, 2015, 184:336-343. doi: 10.1016/j.biortech.2014.10.093 [32] LI X, PRIYANTO D E, ASHIDA R, MIURA K. Two-Stage Conversion of Low-Rank Coal and Biomass into Liquid Fuel under Mild Condition[C]. 29th International Pittsburgh Coal Conference, Pittsburgh USA. 2012. https://www.researchgate.net/publication/275233397_Two-Stage_Conversion_of_Low-Rank_Coal_or_Biomass_into_Liquid_Fuel_under_Mild_Condition [33] SKODRAS G, GRAMMELIS P, BASINAS P, KAKARAS E, SAKELLAROPOULOS G. Pyrolysis and combustion characteristics of biomass and waste-derived feedstock[J]. Ind Eng Chem Res, 2006, 45:3791-3799. doi: 10.1021/ie060107g [34] PIPATMANOMAI S, FUNGTAMMASAN B, BHATTACHARYA S. Characteristics and composition of lignites and boiler ashes and their relation to slagging:The case of Mae Moh PCC boilers[J]. Fuel, 2009, 88:116-123. doi: 10.1016/j.fuel.2008.08.007 [35] KARAGOZ S, BHASKAR T, MUTO A, SAKATA Y, OSHIKI T, KISHIMOTO T. Low-temperature catalytic hydrothermal treatment of wood biomass:Analysis of liquid products[J]. Chem Eng J, 2005, 108:127-137. doi: 10.1016/j.cej.2005.01.007 [36] MATSUI T, NISHIHARA A, UEDA C, OHTSUKI M, IKENAGA N, SUZUKI T. Liquefaction of micro-algae with iron catalyst[J]. Fuel, 1997, 76:1043-1048. doi: 10.1016/S0016-2361(97)00120-8 [37] ELLIOTT D C, BECKMAN D, BRIDGWATER A V, DIEBOLD J P, GEVERT S B, SOLANTAUSTA Y. Developments in direct thermochemical liquefaction of biomass:1983-1990[J]. Energy Fuels, 1991, 5:399-410. doi: 10.1021/ef00027a008 -





 下载:
下载: