Composition and structural characteristics of soluble organic species in Baiyinhua lignite
-
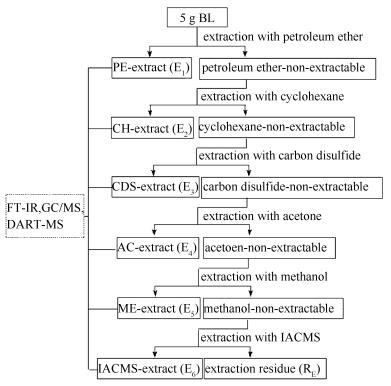
摘要: 依次采用石油醚、环己烷、二硫化碳、丙酮、甲醇和等体积的丙酮/二硫化碳混合溶剂对白音华褐煤(BL)进行分级萃取得到萃取物(E1-E6),利用甲醇、甲苯和等体积的甲醇/甲苯混合溶剂对萃取残渣进行连续热溶得到热溶物(SP1-SP3)。利用傅里叶变换红外光谱(FT-IR)、气相色谱质谱(GC/MS)和实时直接分析-质谱(DART-MS)对萃取物和热溶物的组成和结构特征进行了分析。BL总的萃取物产率和热溶物产率分别为9.37%和21.84%。E5和E6的FT-IR谱图中有较强的羟基吸收峰,而E1的FT-IR谱图中脂肪C-H的伸缩振动峰强度明显高于其他萃取物;三种溶剂热溶物的FT-IR谱图相似,但SP1和SP3的FT-IR谱图中羟基吸收峰较强。E1和E5中GC/MS可检测化合物分别以烷烃和芳烃为主,E5中还含有较多的含氧化合物;三种热溶物中化合物均以烷烃和芳烃为主,SP3中酚类、酮类和酯类等含氧化合物的含量较高。DART-MS可检测出萃取物和热溶物中GC/MS无法检测出的较强极性和难挥发化合物;热溶物中含量较高的化合物的碳数和等价双键数(Double bond equivalent,DBE)分别集中分布在10-25和2-16,并且DBE随碳数增加而增加。Abstract: Baiyinhua lignite was sequentially extracted with petroleum ether, cyclohexane, carbon disulfide, acetone, methanol and isometric acetone/carbon disulfide mixed solvent affording extracts, i.e. E1-E6, then extraction residue was sequentially thermal dissolved using methanol, toluene and isomeric methanol/toluene mixed solvent affording soluble portions (SPs), i.e.SP1-SP3. The composition and structural characteristics of extracts and SPs were characterized with Fourier transform infrared (FT-IR) spectroscopy, gas chromatography/mass spectrometer (GC/MS), direct analyze in real time mass spectrometer (DART-MS). The total extracts yield and SPs yield of BL are 9.37% and 21.84%, respectively. There exist strong adsorption peaks ascribed to hydroxyl in the FT-IR spectra of E1 and E6, while the intensity of adsorption peaks ascribed to aliphatic C-H in the FT-IR spectrum of E1 is obviously higher than that of other extracts. There are similar FT-IR spectra among those 3 SPs, whereas the intensity of adsorption peak originated from hydroxyl in the FT-IR spectra of SP1 and SP3 is higher than those of SP2. The compounds GC/MS detected in E1 and E5 are dominated with alkanes and arenes, respectively, and the content of oxygen-containing compounds (OCCs) in E5 are more than that in E1. The compounds in the 3 SPs are composed of alkanes and arenes, while the contents of OCCs, such as phenols, ketones and esters, in SP3 are higher than those in SP1 and SP2. Many compounds with high polarity and low volatility which could not identified by GC/MS were detected using DART-MS. The carbon number and double bond equivalent (DBE) of the compounds with high content in SPs mainly distribute in 10-25 and 2-16, respectively, and the DBE increases with carbon number increasing.
-
Key words:
- lignite /
- solvent extraction /
- thermal dissolution /
- GC/MS /
- DART-MS
-
表 1 白音华褐煤的工业分析和元素分析
Table 1 Proximate and ultimate analyses of BL
Proximate analysis w/% Ultimate analysis wdaf/% Mad Ad Vdaf C H N S Oa 20.40 7.55 41.64 59.94 5.24 1.82 2.27 30.73 a:by difference 表 2 白音华褐煤的萃取物和热溶物产率
Table 2 Yields of extracts and SPs of BL
Extract Yield wdaf/% SP Yield wdaf/% E1 0.81 SP1 9.29 E2 0.09 SP2 6.62 E3 0.19 SP3 5.93 E4 0.73 E5 7.02 E6 0.53 Total 9.37 total 21.84 -
[1] 李凡, 张永发, 谢克昌.平朔烟煤大分子结构中的小分子相研究[J].燃料化学学报, 1993, 21(3):293-297. http://www.cnki.com.cn/Article/CJFDTOTAL-RLHX199303009.htmLI Fan, ZHANG Yong-fa, XIE Ke-chang. Study on small molecular phase in macromolecules of Pingshuo bituminous coal[J]. J Fuel Chem Technol, 1993, 21(3):293-297. http://www.cnki.com.cn/Article/CJFDTOTAL-RLHX199303009.htm [2] MOSHFIQUR R, ARUNKUMAR S, RAJENDER G. Production and characterization of ash-free coal from low-rank Canadian coal by solvent extraction[J]. Fuel Process Technol, 2013, 115(11):88-98. [3] 张代钧, 王飞, 李小鹏, 鲜学福.溶剂萃取对烟煤孔隙结构和粒度的影响[J].燃料化学学报, 2004, 32(1):18-22. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract16732.shtmlZHANG Dai-jun, WANG Fei, LI Xiao-peng, XIAN Xue-fu. Effect of solvent extraction on pore character and granularity of bituminous coal[J]. J Fuel Chem Technol, 2004, 32(1):18-22. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract16732.shtml [4] SHUI H F, WANG Z C, GAO J S. Examination of the role of CS2 in the CS2/NMP mixed solvents to coal extraction[J]. Fuel Process Technol, 2006, 87(3):185-190. doi: 10.1016/j.fuproc.2004.11.017 [5] JAROSLAV C, HELENA P. Chemical changes of coal matter after treatment with ethylenediamine[J]. Fuel Process Technol, 1991, 27(3):307-318. doi: 10.1016/0378-3820(91)90055-H [6] YUNNUS O, KADIUM C. Low temperature extractability and solvent swelling of Turkish lignites[J]. Fuel Process Technol, 1997, 53(1/2):81-97. [7] LIU F J, WEI F J, WEI X Y, GUI J, LI P, WANG Y G, LI W T, ZONG Z M, FAN X, ZHAO Y P. Characterization of organonitrogen species in Xianfeng lignite by sequential extraction and ruthenium ion-catalyzed oxidation[J]. Fuel Process Technol, 2014, 126(5):199-206. [8] YOSHIDA T, TAKANOHASHI T, SANKANISHI K, SAITO I, FUJITA M, MASHIMO K. The effect of extraction condition on 'HyperCoal' production (1)-under room-temperature filtration[J]. Fuel, 2002, 81(11/12):1463-1469. [9] LU H Y, WEI X Y, YU R, PENG Y L, QI X Z, QIE L M, WEI Q, LV J, ZONG Z M, ZHAO W, ZHAO Y P, NI Z H, WU L. Sequential thermal dissolution of Huolinguole lignite in methanol and ethanol[J]. Energy Fuels, 2011, 25(6):2741-2745. doi: 10.1021/ef101734f [10] BAI Z H, ZHAO Y P, HUANG H D, YANG W H, ZHANG X Z, MU J J, ZHANG P L, SHI Y W. Research status and prospect of lignite upgrading technology in China[J]. Clean Coal Technol, 2013, 19(6):25-29. [11] ZHAO Y P, XIAO J, DING M, EDDINGS E G, WEI X Y, FAN X, ZONG Z M. Sequential extraction and thermal dissolution of Baiyinhua lignite in isometric CS2/acetone and toluene/methanol binary solvents[J]. Energy Fuels, 2016, 30(1):47-53. doi: 10.1021/acs.energyfuels.5b01775 [12] COOKE N E, FULLER O M, GAIKWAD R P. FT-IR spectroscopic analysis of coals and coal extracts[J]. Fuel, 1986, 65(9):1254-1260. doi: 10.1016/0016-2361(86)90238-3 [13] CODY R B, LARAMEE J A, DURST H D. Versatile new lon source for the analysis of materials in open air under ambient conditions[J]. Anal Chem, 2005, 77(8):2297-2302. doi: 10.1021/ac050162j -





 下载:
下载: