Preparation of a novel solid Lewis acid Ce3+-Ti4+-SO42-/MWCNTs and its application in the synthesis of biodiesel from esterification reaction
-
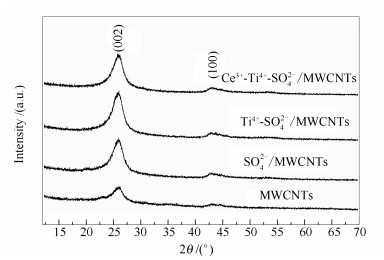
摘要: 采用高温浸渍法,通过Ce3+、Ti4+和浓硫酸磺化反应对多壁纳米碳管进行了改性处理,制备了Lewis酸型固体酸催化剂Ce3+-Ti4+-SO42-/MWCNTs,并采用透射电镜、拉曼光谱、X射线光电子能谱、吡啶吸附红外光谱、X射线荧光光谱、X射线衍射光谱和NH3程序升温脱附等多种测试技术对催化剂的物理化学特性和结构特征进行了表征。以Ce3+-Ti4+-SO42-/MWCNTs为油酸与甲醇经酯化反应合成生物柴油的催化剂,对其催化性能进行了研究。结果表明,当醇油物质的量比为12:1,催化剂与反应物质量比为1%,反应温度为65℃,反应5 h,油酸转化率为93.4%。催化剂Ce3+-Ti4+-SO42-/MWCNTs在重复使用八次后,油酸的转化率仍为80.8%,由此表明其具有较高的催化活性和稳定性。高催化活性和稳定性是因为,纳米碳管的C 1s结合能较一般炭材料低,使得电子在其管状结构中的流动和逃逸非常容易,从而有助于负载于纳米碳管之上的活性组分之间发生强烈的相互作用,最终促使Ce3+和Ti4+分别与SO42-形成稳定的配位键,增大催化剂的晶化程度,并使SO42-与纳米碳管结合的更加牢固,增强了催化剂的稳定性,减少了催化剂中活性组分的流失。最后,由于SO42-与Ce3+的强相互作用,在不增加纳米碳管表面缺陷的情况下,改变了Ti4+-SO42-中表面原子的化学状态,使得S6+离子和Ti4+离子的吸电子能力增加,使催化剂以Lewis酸性活性位为主,避免了SO42-/MWCNTs因为以Brönsted酸位为主,而在富含水的反应介质中,由于水合反应而降低其催化活性的现象发生。
-
关键词:
- 生物柴油 /
- 多壁纳米碳管 /
- Lewis酸型固体酸 /
- 酯化反应 /
- 配位键
Abstract: The Ce3+-Ti4+-SO42-/MWCNTs catalyst was prepared from the modification treatment of multi walled carbon nanotubes by concentrated sulfuric acid, Ce3+ and Ti4+ through the employing of high temperature impregnation method. Physicochemical properties and structural characteristics of the obtained-catalysts were characterized by means of transmission electron microscopy, Raman spectroscopy, X-ray photoelectron spectroscopy, pyridine adsorption FT-IR spectra, X-ray fluorescence spectroscopy, X-ray diffraction and NH3 temperature programmed desorption. The catalytic activity of Ce3+-Ti4+-SO42-/MWCNTs for the synthesis of biodiesel from the esterification of methanol and oleic acid was investigated. The influence of SO42-/MWCNTs, which was resulted from the addition of Ce3+ and Ti4+, on the structure and catalytic activity was cleared based on the above structure characterization and catalytic activity investigation. The results showed that the conversion of oleic acid reached 93.4% after 5 h reaction at 65℃, when the catalyst/reactants was 1% and the molar ratio of methanol/oleic acid was 12:1. The conversion of oleic acid was 80.8% after the Ce3+-Ti4+-SO42-/MWCNTs were cycled for eight times. Therefore, it can be concluded that this catalyst has high catalytic activity and stability. The high catalytic activity and stability can be explained as follows:the C 1s binding energy of carbon nanotube is much lower than other carbon materials, resulting in easy flow and escape of the electrons in the tubular structure. Thus, the strong interactions will occur among the active groups that have been loaded on the carbon nanotube, which impels Ce3+ and Ti4+ to respectively form stable coordination bonds with SO42-, increases the crystallization degree of the Ce3+-Ti4+-SO42-/MWCNTs catalyst and the active acid sites without increasing the surface defects, and the combination of SO42- and MWCNTs was more stable. In addition, the chemical state of surface atom on the Ti-SO42- was changed due to the strong interaction between SO42- and Ce3+, which strengthened the electron withdrawing ability of S6+ and Ti4+ ions and enhanced the acidity strength of Lewis acid with changing of the acid type. Hence, the Ce3+-Ti4+-SO42-/MWCNTs will be composed by Lewis acid sites mainly, which is favorable for avoiding the occurrence of hydration of acid active sites for the SO42-/MWCNTs catalyst because it was composed of Brönsted acid sites mainly.-
Key words:
- biodiesel /
- carbon nanotube /
- solid Lewis Acid /
- esterification /
- coordination bond
-
图 8 SO42-/MWCNTs、Ti4+-SO42-/MWCNTs、Ce3+-SO42-/MWCNTs、Ce3+-Ti4+-SO42-/MWCNTs的催化酯化反应活性比较
Figure 8 Comparison of catalytic activity of SO42-/MWCNTs, Ti4+-SO42-/MWCNTs, Ce3+-SO42-/MWCNTs, Ce3+-Ti4+-SO42- /MWCNTs in the esterification
reaction conditions: mass ratio of catalyst to reactants is 0.5%, the mol ratio of methanol to oleic acid is 12: 1, reaction temperature is 65 ℃
-
[1] 梁金花, 任晓乾, 王锦堂, 姜岷, 李振江.双核碱性离子液体催化棉籽油酯交换制备生物柴油[J].燃料化学学报, 2010, 38(3):275-280. doi: 10.1016/S1872-5813(10)60033-3LIANG Jin-hua, REN Xiao-qian, WANG Jin-tang, JIANG min, LI Zhen-jiang. Preparation of biodiesel by transesterification from cottonseed oil using the basic dication ionic liquids as catalyst[J]. J Fuel Chem Technol, 2010, 38(3):275-280. doi: 10.1016/S1872-5813(10)60033-3 [2] HOSSEINI S, JANAUN J, CHOONG T S Y. Feasibility of honeycomb monolith supportedsugar catalyst to produce biodiesel from palm fattyacid distillate (PFAD)[J]. Process Saf Environ Prot, 2015, 98:285-295. doi: 10.1016/j.psep.2015.08.011 [3] SHYAMSUNDAR M, SHAMSHUDDIN S Z M, ANIZ C U. Cordierite honeycomb monoliths coated with zirconia and its modified forms for biodiesel synthesis from pongamiaglabra[J]. J Am Oil Chem Soc, 2015, 92(3):335-344. doi: 10.1007/s11746-015-2609-4 [4] KAUR N, ALI A. Preparation and application of Ce/ZrO2-TiO2/SO42-as solid catalyst for the esterification of fatty acids[J]. Renewable Energy, 2015, 64(11):6392-6395. [5] 舒庆, 侯小鹏, 刘峰生, 唐国强, 许宝泉, 张彩霞.稀土镧改性磷钨杂多酸盐催化油酸与甲醇酯化反应合成生物柴油活性研究[J].有色金属科学与工程, 2016, 7(3):131-136. http://www.cnki.com.cn/Article/CJFDTOTAL-JXYS201603023.htmSHU Qing, HOU Xiao-peng, LIU Feng-sheng, TANG Guo-qiang, XU Bao-quan, ZHANG Cai-xia. Studies on the catalytic activity of La-modified phosphotungstic heteropoly acid salt in the synthesis of biodiesel from the esterification of methanol and oleci acid[J]. Nonfer Metal Sci Eng, 2016, 7(3):131-136. http://www.cnki.com.cn/Article/CJFDTOTAL-JXYS201603023.htm [6] SHU Q, ZHANG Q, XU G, WANG J F. Preparation of biodiesel using s-MWCNT catalysts and the coupling of reaction and separation[J]. Food Bioprod Process, 2009, 87(3):164-170. doi: 10.1016/j.fbp.2009.01.004 [7] JUAN J C, JIANG Y J, MENG X J, CAO W L, YARMO M A, ZHANG J C. Supported zirconium sulfate on carbon nanotubes as water-tolerant solid acid catalyst[J]. Mater Res Bull, 2007, 42(7):1278-1285. doi: 10.1016/j.materresbull.2006.10.017 [8] IGLESIAS J, MELERO J A, MORALES G, MORENO J, SEGURA Y, PANIAGUA M, CAMBRA A, HERNÁNDEZ B. Zr-SBA-15 lewis acid catalyst:Activity in meerwein ponndorf verley reduction[J]. Catalysts, 2015, 5(4):1911-1927. doi: 10.3390/catal5041911 [9] GUAN Q Q, SHANG H, LIU J, NING P. Biodiesel from transesterification at low temperature by AlCl3catalysis in ethanol and carbon dioxide as cosolvent:Process, mechanism and application[J]. Appl Energy, 2016, 164:380-386. doi: 10.1016/j.apenergy.2015.11.029 [10] MELERO J A, IGLESIAS J, MORALES G. Heterogeneous acid catalysts for biodiesel production:current status and future challenges[J]. Green Chem, 2009, 11(11):1285-1308. https://www.researchgate.net/publication/244550800_Heterogeneous_acid_catalysts_for_biodiesel_production_Current_status_and_future_challenges [11] REINOSO D M, DAMIANI D E, TONETTO G M. Efficient production of biodiesel from low-cost feedstock using zinc oleate as catalyst[J]. Fuel Process Technol, 2015, 134:26-31. doi: 10.1016/j.fuproc.2015.03.003 [12] CHERYL-LOW Y L, THEAM K L, LEE H V. Alginate-derived solid acid catalyst for esterification of low-cost palm fatty acid distillate[J]. Energ Convers Manage, 2015, 106:932-940. doi: 10.1016/j.enconman.2015.10.018 [13] ALMEIDA R M D, NODA L K, GONALVES N S, MENEGHETTI S M P, MENEGHETTI M R. Transesterification reaction of vegetable oils, using superacid sulfated TiO2-base catalysts[J]. Appl Catal A:Gen, 2008, 347(1):100-105. doi: 10.1016/j.apcata.2008.06.006 [14] 侯凯军, 孟明, 邹志强, 吕倩.掺杂La3+对纳米Au/TiO2催化剂结构和性能的影响[J].无机化学学报, 2007, 23(9):1538-1544. http://www.wjhxxb.cn/wjhxxbcn/ch/reader/view_abstract.aspx?file_no=20070907HOU Kai-Jun, MENG Ming, ZOU Zhi-Qiang, LV Qian. Effect of La3+ doping on the structures and performance of nano-structured Au/TiO2 catalysts[J]. Chin J Inorg Chem, 2007, 23(9):1538-1544. http://www.wjhxxb.cn/wjhxxbcn/ch/reader/view_abstract.aspx?file_no=20070907 [15] ZHANG X H, TANG Q Q, YANG D, HU J H. Preparation of poly (p-styrenesulfonic acid) grafted multi-walled carbonnanotubes and their application as a solid-acid catalyst[J]. Mater Chem Phys, 2011, 126(1):310-313. https://www.researchgate.net/profile/Bian_Gang/publication/283537763_A_novel_polyp-styrenesulfonic_acid_grafted_carbon_nanotubegraphene_oxide_architecture_with_enhanced_catalytic_performance_for_the_synthesis_of_benzoate_esters_and_fatty_acid_alkyl_esters/links/5649b43b08ae9f9c13ec7b02.pdf [16] JUMI Y, JI S I, YOUNG S L, HYUNG I K. Effect of oxyfluorination on electromagnetic interference shielding behavior of MWCNT/PVA/PAAc composite microcapsules[J]. Eur Polym J, 2010, 46(5):900-909. doi: 10.1016/j.eurpolymj.2010.02.005 [17] REDDY G K, HE J, THIEL S W, PINTO N G, SMIRNIOTIS P G. Sulfur-tolerant Mn-Ce-Ti sorbents for elemental mercury removal from flue gas:Mechanistic investigation by XPS[J]. J Phys Chem C, 2015, 119(16):8634-8644. doi: 10.1021/jp512185s [18] LI Z J, DENG S B, YU G, HUANG J, LIM V C. As (Ⅴ) and As (Ⅲ) removal from water by a Ce-Ti oxide adsorbent:Behavior and mechanism[J]. Chem Eng J, 2010, 161(1/2):106-113. https://www.researchgate.net/publication/223927387_AsV_and_AsIII_Removal_from_Water_by_a_Ce-Ti_Oxide_Adsorbent_Behavior_and_Mechanism?_sg=lC3UM9wFYIZW1jR0EPPVnYeEuqsTe8qHTgGVRKwtxaM_wZCuuXc9r8AQ-ZTvhxvNsHPH3LT3qcoVTSRPY1dQjQ [19] LAN L, CHEN S, ZHAO M, GONG M, CHEN Y. The effect of synthesis method on the properties and catalyticperformance of Pd/Ce0.5Zr0.5O2-Al2O3 three-way catalyst[J]. J Mol Catal A:Chem, 2014, 394(10):10-21. [20] SHU Q, NAWAZ Z, GAO J X, WANG J F. Synthesis of biodiesel from a model waste oil feedstock using a carbon-based solid acid catalyst:Reaction and separation[J]. Bioresource Technol, 2010, 101(14):5374-5384. doi: 10.1016/j.biortech.2010.02.050 -





 下载:
下载: