MoO3-SnO2 catalyst prepared by hydrothermal synthesis method for dimethyl ether catalytic oxidation
-
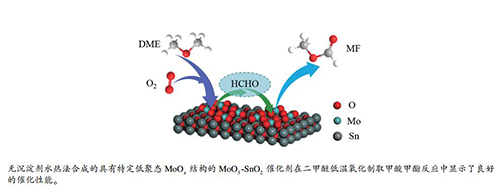
摘要: 采用无沉淀剂水热法一步合成了MoO3-SnO2复合金属氧化物催化剂,通过调变Mo/Sn物质的量比,考察了催化剂上活性组分MoOx分散程度对二甲醚(DME)低温氧化生成甲酸甲酯(MF)反应性能的影响。当Mo/Sn=1:2,反应条件为150℃时,催化剂表现出较好的催化性能,DME转化率为22.0%,MF选择性达到77.6%。实验中采用TEM、XRD、Raman、FT-IR、NH3-TPD及H2-TPR等表征对催化剂晶体结构及表面性质进行了分析。结果发现,Mo/Sn物质的量比变化会对催化剂晶体结构产生显著影响,钼氧化物在SnO2表面形成不同分散程度的MoOx结构,这种钼氧化物结构的变化进一步影响了催化剂表面的酸性及氧化还原性,是造成催化性能差异的主要原因。Abstract: MoO3-SnO2 composite metal oxide catalyst was synthesized by one-step hydrothermal synthesis method without precipitant. The effect of MoOx dispersion state on the catalytic performance of MoO3-SnO2 catalysts with different Mo/Sn molar ratios was investigated for dimethyl ether (DME) low-temperature oxidation to methyl formate (MF). MF selectivity reaches 77.6% with DME conversation of 22.0% over Mo1Sn2 at 150 ℃. The physiochemical properties of these catalysts were characterized by TEM, XRD, Raman, FT-IR, NH3-TPD and H2-TPR. The results showed that the addition of SnO2 into MoO3 affected the crystal structure of catalysts, forming MoOx species with different degree of dispersion on the surface of SnO2. The special architecture of MoOx-SnO2 plays a major role in modifying the acidity and the oxidizability of MoO3-SnO2 catalysts, leading to the obvious differences on catalytic activity.
-
Key words:
- hydrothermal synthesis /
- dimethyl ether /
- selective oxidation /
- methyl formate /
- MoOx
-
表 1 不同Mo/Sn比例的MoO3-SnO2催化剂对二甲醚选择性氧化制备甲酸甲酯的影响
Table 1 Effects of different Mo/Sn mole ratio on the reaction of DME to MF over MoO3-SnO2 catalysts
Catalyst DME conversion x/% Cmol-selectivity s/% MF CH3OH HCHO DMM CO MoO3 3.4 0 98.8 1.2 0 0 Mo3Sn1 6.6 54.3 34.0 5.4 2.2 4.1 Mo2Sn1 8.5 67.0 25.8 3.9 0.2 3.1 Mo1Sn1 17.1 75.9 6.3 0 3.9 13.9 Mo1Sn2 22.0 77.6 8.6 0 1.4 12.4 Mo1Sn3 15.6 77.1 9.5 0 0 13.4 Mo1Sn4 10.6 76.4 23.2 0.4 0 0 Mo1Sn5 6.6 71.1 28.0 0.9 0 0 Mo1Sn10 4.2 41.9 58.1 0 0 0 SnO2 2.8 0 100 0 0 0 reaction conditions: tR=150 ℃, atmospheric pressure, n(DME):n(O2)=1:1, GHSV=1800 h-1; MF: HCOOCH3, MeOH: CH3OH, FA: HCHO, DMM: CH3OCH2OCH3 -
[1] SEMELSBERGER T A, BORUP R L, GREENE H L. Dimethyl ether(DME) as an alternative fuel[J]. J Power Sources, 2006, 156(2):497-511. doi: 10.1016/j.jpowsour.2005.05.082 [2] XU M T, LUNSFORD J H, GOODMAN D W, BHATTACHARYYA A. Synthesis of dimethyl ether (DME) from methanol over solid-acid catalysts[J]. Appl Catal A:Gen, 1997, 149(2):289-301. doi: 10.1016/S0926-860X(96)00275-X [3] TAN Y S, XIE H J, CUI H T, HAN Y Z, ZHONG B. Modification of Cu-based methanol synthesis catalyst for dimethyl ether synthesis from syngas in slurry phase[J]. Catal Today, 2005, 104(1):25-29. http://cn.bing.com/academic/profile?id=a3050a170c64e3903ca6807b9414d6db&encoded=0&v=paper_preview&mkt=zh-cn [4] SUN J, YANG G H, YONEYAMA Y, TSUBAKI N. Catalysis chemistry of dimethyl ether synthesis[J]. ACS Catal, 2014, 4(10):3346-3356. doi: 10.1021/cs500967j [5] 孙明, 余林, 孙长勇, 宋一兵, 孙建.二甲醚的应用及下游产品开发[J].精细化工, 2003, 20(11):695-699. doi: 10.3321/j.issn:1003-5214.2003.11.017SUN Ming, YU Lin, SUN Chang-yong, SONG Yi-bing, SUN Jian. Application of Dimethyl ether and development of its downstream products[J]. Fine Chem, 2003, 20(11):695-699. doi: 10.3321/j.issn:1003-5214.2003.11.017 [6] ZHANG Q D, TAN Y S, LIU G B, ZHANG J F, HAN Y Z. Rhenium oxide-modified H3PW12O40/TiO2 catalysts for selective oxidation of dimethyl ether to dimethoxy dimethyl ether[J]. Green Chem, 2014, 16(11):4708-4715. doi: 10.1039/C4GC01373E [7] ZHANG Q D, WANG W F, ZHANG Z Z, HAN Y Z, TAN Y S. Low-temperature oxidation of dimethyl ether to polyoxymethylene dimethyl ethers over CNT-supported rhenium catalyst[J]. Catalysts, 2016, 6(3):43. doi: 10.3390/catal6030043 [8] GAO X J, WANG W F, GU Y Y, ZHANG Z Z, ZHANG J F, ZHANG Q D, TSUBAKI N, HAN Y Z, TAN Y S. Synthesis of polyoxymethylene dimethyl ethers from dimethyl ether direct oxidation over carbon-based catalysts[J]. ChemCatChem, 2018, 10(1):273-279. doi: 10.1002/cctc.v10.1 [9] ZHANG Z Z, ZHANG Q D, JIA L Y, WANG W F, XIAO H, HAN Y Z, TSUBAKI N, TAN Y S. Effects of MoO3 crystalline structure of MoO3-SnO2 catalysts on selective oxidation of glycol dimethyl ether to 1, 2-propandiol[J]. Catal Sci Technol, 2016, 6(6):1842-1849. doi: 10.1039/C5CY00894H [10] HUANG X M, LIU J L, CHEN J L, XU Y D, SHEN W J. Mechanistic study of selective oxidation of dimethyl ether to formaldehyde over alumina-supported molybdenum oxide catalyst[J]. Catal Lett, 2006, 108(1/2):79-86. http://cn.bing.com/academic/profile?id=7fd08a98c45253b3194327f57adaf6d4&encoded=0&v=paper_preview&mkt=zh-cn [11] 高秀娟, 王文峰, 张振洲, 张清德, 谭猗生, 韩怡卓.二甲醚氧化制聚甲氧基二甲醚的研究进展[J].石油化工, 2017, 46(2):143-150. http://d.old.wanfangdata.com.cn/Periodical/syhg201702001GAO Xiu-juan, WANG Wen-feng, ZHANG Zhen-zhou, ZHANG Qing-de, TAN Yi-sheng, HAN Yi-zhuo. Progresses in synthesis of polymethylene dimethyl ethers from dimethyl ether[J]. Petrochem Technol, 2017, 46(2):143-150. http://d.old.wanfangdata.com.cn/Periodical/syhg201702001 [12] 汪多仁.甲酸甲酯的生产发展与应用前景[J].石油与天然气化工, 1998, 27(3):149-151. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK199800540043WANG Duo-ren. Production development and application prospects of methyl formate[J]. Chem Eng Oil Gas, 1998, 27(3):149-151. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK199800540043 [13] 周寿祖.甲酸甲酯的生产技术和应用前景[J].化工科技市场, 2003, 26(2):13-18. http://d.old.wanfangdata.com.cn/Periodical/hgkjsc200302004ZHOU Shou-zu. Production technology and application foreground of methyl formate[J]. Chem Technol Market, 2003, 26(2):13-18. http://d.old.wanfangdata.com.cn/Periodical/hgkjsc200302004 [14] AI M. The production of methyl formate by the vapor-phase oxidation of methanol[J]. J Catal, 1982, 77(1):279-288. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=e3f3f3492468b3d76c12c7ba1bdb1945 [15] AI M. The reaction of formaldehyde on various metal-oxide catalysts[J]. J Catal, 1983, 83(1):141-150. doi: 10.1016-0021-9517(83)90037-4/ [16] LIU H C, CHEUNG P, IGLESIA E. Structure and support effects on the selective oxidation of dimethyl ether to formaldehyde catalyzed by MoOx domains[J]. J Catal, 2003, 217(1):222-232. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=180d6d3f82f0282854bdd2714ae015e2 [17] LIU H C, CHEUNG P, IGLESIA E. Effects of Al2O3 support modifications on MoOx and VOx catalysts for dimethyl ether oxidation to formaldehyde[J]. Phys Chem Chem Phys, 2003, 5(17):3795-3800. doi: 10.1039/b302776g [18] LIU G B, ZHANG Q D, HAN Y Z, TSUBAKI N, TAN Y S. Selective oxidation of dimethyl ether to methyl formate over trifunctional MoO3-SnO2 catalyst under mild conditions[J]. Green Chem, 2013, 15(6):1501-1504. doi: 10.1039/c3gc40279g [19] LIU G B, ZHANG Q D, HAN Y Z, TSUBAKI N, TAN Y S. Effects of the MoO3 structure of Mo-Sn catalysts on dimethyl ether oxidation to methyl formate under mild conditions[J]. Green Chem, 2015, 17(2):1057-1064. doi: 10.1039/C4GC01591F [20] 刘广波, 张清德, 韩怡卓, 椿范立, 谭猗生. MoO3-SnO2催化剂上二甲醚低温氧化高选择性制备甲酸甲酯[J].燃料化学学报, 2013, 41(2):223-227. doi: 10.3969/j.issn.0253-2409.2013.02.015LIU Guang-bo, ZHANG Qing-de, HAN Yi-zhuo, TSUBAKI Noritatsu, TAN Yi-sheng. Low-temperature oxidation of dimeyhyl ether to methyl formate with high selectivity over MoO3-SnO2 catalysts[J]. J Fuel Chem Technol, 2013, 41(2):223-227. doi: 10.3969/j.issn.0253-2409.2013.02.015 [21] ZHANG Z Z, ZHANG Q D, JIA L Y, WANG W F, ZHANG T, HAN Y Z, TSUBAKI N, TAN Y S. Effects of tetrahedral molybdenum oxide species and MoOx domains on the selective oxidation of dimethyl ether under mild conditions[J]. Catal Sci Technol, 2016, 6(9):2975-2983. doi: 10.1039/C5CY01569C [22] ZHANG Z, ZHANG Q, JIA L, WANG W, TIAN S P, WANG P, XIAO H, HAN Y, TSUBAKI N, TAN Y. The effects of the Mo-Sn contact interface on the oxidation reaction of dimethyl ether to methyl formate at a low reaction temperature[J]. Catal Sci Technol, 2016, 6(15):6109-6117. doi: 10.1039/C6CY00460A [23] DEVAN R S, PATIL R A, LIN J H, MA Y R. One-dimensional metal-oxide nanostructures:Recent developments in synthesis, characterization, and applications[J]. Adv Funct Mater, 2012, 22(16):3326-3370. doi: 10.1002/adfm.v22.16 [24] 赵延霞.水热法合成一维纳米级MoO3微粉[J].中国钨业, 2012, 27(3):25-29. doi: 10.3969/j.issn.1009-0622.2012.03.007ZHAO Yan-xia. Synthesis the one-dimensional nano-size MoO3 via acidification for hydrolyzing[J]. China Tungsten Ind, 2012, 27(3):25-29. doi: 10.3969/j.issn.1009-0622.2012.03.007 [25] 张建荣, 高濂.水热法合成纳米SnO2粉体[J].无机材料学报, 2004, 19(5):1177-1180. doi: 10.3321/j.issn:1000-324X.2004.05.034ZHANG Jian-rong, GAO Lian. Hydrothermal synthesis of tin oxide nanoparticles[J]. J Inorg Mater, 2004, 19(5):1177-1180. doi: 10.3321/j.issn:1000-324X.2004.05.034 [26] GONCALVES F, MEDEIROS P R S, EON J G, APPEL L G. Active sites for ethanol oxidation over SnO2-supported molybdenum oxides[J]. Appl Catal A:Gen, 2000, 193(1/2):195-202. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=3e9f3819bf721b2b4608bb2ff36b370f [27] ROUTRAY K, ZHOU W, KIELY C J, GRUNERT W, WACHS I E. Origin of the synergistic interaction between MoO3 and iron molybdate for the selective oxidation of methanol to formaldehyde[J]. J Catal, 2010, 275(1):84-98. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=acb38cb74d8ce4f1f2a27a98da4faf25 [28] HERRMANN J M, VILLAIN F, APPEL L G. Characterization of Mo-Sn-O system by means of Raman spectroscopy and electrical conductivity measurements[J]. Appl Catal A:Gen, 2003, 240(1/2):177-182. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=ce15e10e291573e3004eb47ed48b1907 [29] LAKSHMI L J, ALYEA E C. ESR, FT-Raman spectroscopic and ethanol partial oxidation studies on MoO3/SnO2 catalysts made by metal oxide vapor synthesis[J]. Catal Lett, 1999, 59(1):73-77. https://www.onacademic.com/detail/journal_1000034816457110_b8a9.html [30] HABER J, LALIK E. Catalytic properties of MoO3 revisited[J]. Catal Today, 1997, 33(1/3):119-137. https://www.sciencedirect.com/science/article/abs/pii/S0920586196001071 [31] NIWA M, IGARASHI J. Role of the solid acidity on the MoO3 loaded on SnO2 in the methanol oxidation into formaldehyde[J]. Catal Today, 1999, 52(1):71-81. https://www.sciencedirect.com/science/article/abs/pii/S0920586199000644 [32] FENG Q Y, YAO S L. Infrared study of ZrO2 surface sites using adsorbed probe molecules. 2. Dimethyl ether adsorption[J]. J Phys Chem B, 2000, 104(47):11253-11257. doi: 10.1021/jp002509m [33] 杜英辉, 许国基.氢气还原金属氧化物[J].原子能科学技术, 1999, 33(4):360-362. doi: 10.3969/j.issn.1000-6931.1999.04.020DU Ying-hui, XU Guo-ji. Hydrogen reduction of metal oxides to metals[J]. Atom Energy Sci Technol, 1999, 33(4):360-362. doi: 10.3969/j.issn.1000-6931.1999.04.020 -





 下载:
下载: