Research on the mechanism of xK/MgAlO hydrotalcite for the catalytic combustion of soot
-
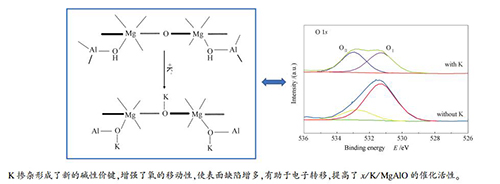
摘要: 通过等体积浸渍法制备了不同K掺杂量的镁铝水滑石复合氧化物(xK/MgAlO),利用X射线衍射光谱及暂态响应、扫描电子显微镜、傅里叶变换红外光谱、X射线光电子能谱及程序升温等技术比较了焙烧和未焙烧的MgAlO形貌结构和晶型的异同,在含硫气氛中研究了K对镁铝水滑石复合氧化物形貌结构和催化碳烟燃烧性能的影响,阐明了反应过程中K掺杂的xK/MgAlO型催化剂降低碳烟起燃温度的关键机制。结果表明,焙烧后的镁铝水滑石3R层状结构消失,出现了尖晶石相,层状结构坍塌变为球形颗粒状;掺杂钾后的催化剂(K/MgAlO)表面活性氧与晶格氧的比例增大,使得氧空位的数量增多,有效提高了催化剂的催化反应活性。在模拟烟气实验中发现掺杂量x=7的(7K/MgAlO)催化剂在含SO2的混合气中使碳烟的起燃温度降低了127℃,且对NOx的转化率显著增强。Abstract: The Mg-Al hydrotalcites used as the support were prepared by precipitation method, and then the catalysts with different amount of doped potassium, (xK/MgAlO) were prepared by impregnation method. The effects of K on the structure and catalytic activity of the xK/MgAlO catalyst were investigated in SO2 containing gases. The key mechanism of K-doped (xK/MgAlO) catalysts to reduce the soot ignition temperature during the reaction was illustrated. The differences of the crystal structure between calcined and uncalcined Mg-Al hydrotalcite were studied by X-ray diffraction, scanning electron microscopy, Fourier transform infrared spectroscopy, and the transient response method. The experimental results showed that the 3R layered structure of Mg-Al hydrotalcite disappeared while the spinel phase appeared, and the layered structure collapsed into spherical particles after calcination. Potassium doping formed more oxygen vacancies that are conducive to the combustion of diesel soot, decreased the temperature of soot combustion from 380 to 253℃ though in SO2 atmosphere and significantly enhanced the conversion efficiency of NOx.
-
Key words:
- Mg-Al hydrotalcite /
- potassium /
- NOx /
- sulfur dioxide /
- oxygen vacancies
-
表 1 实验烟气参数
Table 1 Parameters of the flue gas used in the experiment
Gas φ/mL Total flow /(mL·min-1) SO2 NO CO C3H6 O2 Ar 1 14.7 0.25 1.25 0.125 25 208.7 250 表 2 碳烟的起燃温度
Table 2 Ignition temperature of soot on different catalysts
Catalyst Ignition temperature tig /℃ MgAlO 380 3K/MgAlO 312 5K/MgAlO 297 7K/MgAlO 253 9K/MgAlO 277 表 3 催化剂的晶格参数和比表面积
Table 3 Lattice parameters and specific surface area of the catalysts
Catalyst Lattice parameters /nm ABET /(m2·g-1) a b c d MgAlO 3.444 3.191 5.988 642 113.2 7K/MgAlO 3.479 2.822 3.409 671 51.6 表 4 MgAlO和7K/MgAlO的XPS分析结果
Table 4 XPS analysis results of MgAlO and 7K/MgAlO
Catalyst Surface atomic ratio /% Atomic ratio Mg Al K C OⅠ OⅡ Mg/Al K/(Mg+Al) MgAlO 14.10 6.20 - 22.26 45.13 12.09 2.27 - 7K/MgAlO 8.49 5.98 8.30 25.89 26.49 24.85 1.42 0.57 -
[1] NEEFT J P A, MAKKEE M, MOULIJIN J A. Diesel particulate emission control[J]. Fuel Process Technol, 1996, 47:1-69. doi: 10.1016/0378-3820(96)01002-8 [2] JOHNSON T V. Diesel emission control in review[J]. SAE Int J Fuels Lubr, 2006, 2006-01-0030. http://d.old.wanfangdata.com.cn/OAPaper/oai_doaj-articles_35f11255f1100958c7502ee7a120f177 [3] 贺泓, 翁端, 资新运.柴油车尾气排放污染控制技术综述[J].环境科学, 2007, 28(6):1169-1177. doi: 10.3321/j.issn:0250-3301.2007.06.001HE Hong, WEN Duan, ZI Xin-yun. Diesel emission control technologies:A review[J]. Environ Sci, 2007, 28(6):1169-1177. doi: 10.3321/j.issn:0250-3301.2007.06.001 [4] 王林江, 郭子峰, 吴群英.柴油车尾气净化四效催化技术进展[J].工业催化, 2009, 17(5):3-6. http://d.old.wanfangdata.com.cn/Conference/7111696WANG Lin-jiang, GUO Zi-feng, WU Qun-ying. Advances in four-way catalytic technology for diesel vehicle exhaust gas purification[J]. Ind Catal, 2009, 17(5):3-6. http://d.old.wanfangdata.com.cn/Conference/7111696 [5] 周克斌, 陈宏德, 田群, 沈迪新, 徐晓白. Pd掺杂对Fe, Co系钙钛矿型三效催化剂性能的影响[J].环境化学, 2002, 21:218-223. doi: 10.3321/j.issn:0254-6108.2002.03.002ZHOU Ke-bin, CHEN Hong-de, TIAN Qun, SHEN Di-xin, XU Xiao-bai. Study on the effect of doped chemicals palladium on the performance of Co and Fe series perovskite-type three-way catalysts[J]. Environ Chem, 2002, 21:218-223. doi: 10.3321/j.issn:0254-6108.2002.03.002 [6] JOHNSON T. Diesel engine emissions and their control[J]. Platinum Met Rev, 2008, 52(1):23-37. doi: 10.1595/147106708X248750 [7] 韩小伟, 王英.水滑石及类水滑石材料的合成及应用新进展[J].江苏化工, 2003, 31(2):26-31. http://d.old.wanfangdata.com.cn/Periodical/jshg200302006HAN Xiao-wei, WANG Ying. Progresses in preparation and application of hydrotalcite and hydrotalcite-like materials[J]. Jiangsu Chem Ind, 2003, 31(2):26-31. http://d.old.wanfangdata.com.cn/Periodical/jshg200302006 [8] 赵娜.镁铝系NSR催化剂的制备及催化性能研究[D].昆明: 昆明理工大学, 2014. http://cdmd.cnki.com.cn/Article/CDMD-10674-1014349301.htmZHAO Na. Preparation and catalytic performance of NSR catalysts over magnesium-aluminum[D]. Kunming: Kunming University of Science and Technology, 2014. http://cdmd.cnki.com.cn/Article/CDMD-10674-1014349301.htm [9] TERAOKA Y, KANADA K, KAGAWA S. Synthesis of La-K-Mn-O perovskite-type oxides and their catalytic property for simultaneous removal of NOx and diesel soot particulates[J]. Appl Catal B:Environ, 2001, 34(1):73-78. http://www.sciencedirect.com/science/article/pii/S0926337301002028 [10] ATRIBAK I, SUCH-BASANEZ I, BUENO-LOPEZ A, GARCIA GARCIA A. Catalytic activity of La-modified TiO2 for soot oxidation by O2[J]. Appl Catal A:Gen, 2006, 314(1):81-88. doi: 10.1016/j.apcata.2006.08.002 [11] ZHANG Z L, ZHANG Y X, WANG Z P. Catalytic performance and mechanism of potassium-supported Mg-Al hydrotalcite mixed oxides for soot combustion with O2[J]. J Catal, 2010, 271:12-21. doi: 10.1016/j.jcat.2010.01.022 [12] LI Q, MENG M, ZOU Z Q, LI X G, ZHA Y Q. Simultaneous soot combustion and nitrogen oxides storage on potassium-promoted hydrotalcite-based CoMgAlO catalysts[J]. J Hazard Mater, 2009, 161:366-372. doi: 10.1016/j.jhazmat.2008.03.103 [13] LI Q, MENG M, TSUBAKI N, LI X G, LI Z Q, XIE Y N, HU T D, ZHANG J. Performance of K-promoted hydrotalcite-derived Co MgAlO catalysts used for soot combustion, NOx storage and simultaneous soot-NOx removal[J]. Appl Catal B:Environ, 2009, 91:406-415. doi: 10.1016/j.apcatb.2009.06.007 [14] CHMIELARZA L, JABLON'SKA M, STRUMIN'SKI A, PIWOWARSKA Z, WEGRZYN A, WITKOWSKI S, MICHALIK M. Selective catalytic oxidation of ammonia to nitrogen over Mg-Al, Cu-Mg-Al and Fe-Mg-Al mixed metal oxides doped with noble metals[J]. Appl Catal B:Environ, 2013, 130:152-162. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=258bd32f114cf740585538b055fdadc7 [15] WALSPURGER S, BOELS L, COBDEN P D, ELZINGA G D, HAIJE W G, VANDEN BRINK R W. The crucial role of the K+-aluminium oxide interaction in K+-promoted alumina-and hydrotalcite-based materials for CO2 sorption at high temperatures[J]. ChemSusChem, 2008, 1(7):643-650. doi: 10.1002/cssc.v1:7 [16] ZHANG Z L, ZHANG Y X, SU Q Y, WANG Z P, LI Q, GAO X Y. Determination of intermediates and mechanism for soot combustion with NOx/O2 on potassium-supported Mg Al hydrotalcite mixed oxides by in situ FTIR[J]. Environ Sci Technol, 2010, 44:8254-8258. doi: 10.1021/es102363f [17] ZHU L, WANG X, LIANG C. Catalytic combustion of diesel soot over K2NiF4-type oxides La2-xKxCuO4[J]. J Rare Earth, 2008, 2:254-257. http://d.wanfangdata.com.cn/Periodical/zgxtxb-e200802025 [18] IORDAN A, ZAKI M I, KAPPENSTEIN C. Interfacial chemistry in the preparation of catalytic potassium-modified aluminas[J]. J Chem Soc, Faraday Trans, 1993, 89(14):2527-2536. doi: 10.1039/ft9938902527 [19] 刘坚, 赵震, 徐春明, 王虹, 段爱军. Mn1-x(Li, Ti)xCo2O4尖晶石型复合氧化物的制备、表征与催化性能[J].无机化学学报, 2005, 21(9):1306-1310. doi: 10.3321/j.issn:1001-4861.2005.09.006LIU Jian, ZHAO Zhen, XU Chun-ming, WANG Hong, DUAN Ai-jun. Preparation, characterization and catalytic behavior of Mn1-x(Li, Ti)xCo2O4 spinel-type complex oxides[J]. J Inorg Chem, 2005, 21(9):1306-1310. doi: 10.3321/j.issn:1001-4861.2005.09.006 [20] 李爽, 史翊翔, 杨懿, 朱炫灿, 蔡宁生.钾修饰水滑石吸附剂脱碳性能及颗粒强度实验研究[J].工程热物理学报, 2015, 36(7):1606-1610. http://www.cqvip.com/QK/90922X/201507/665467968.htmlLI Shuang, SHI Yi-xiang, YANG Yi, ZHU Xuan-can, CAI Ning-sheng. Experimental study of CO2 capacity and mechanical Strength of K-promoted hydrotalcite adsorbent[J]. J Eng Thermophy, 2015, 36(7):1606-1610. http://www.cqvip.com/QK/90922X/201507/665467968.html [21] PENG X S, LIN H, SHANGGUAN W F. Surface properties and catalytic performance of La0.8K0.2Cux Mn1-xO3 for simultaneous removal of NOx and soot[J]. Chem Eng Technol, 2007, 30(1):99-104. doi: 10.1002/(ISSN)1521-4125 [22] 彭小圣.催化与高频放电耦合催化同时去除氮氧化物和碳烟[D].上海: 上海交通大学, 2006. http://cdmd.cnki.com.cn/Article/CDMD-10248-2006194503.htmPENG Xiaosheng. Simultaneous removal of NOx and soot by high frequency dielectric barrier discharge and catalysis[D]. Shanghai: Shanghai Jiao Tong University, 2006. http://cdmd.cnki.com.cn/Article/CDMD-10248-2006194503.htm [23] 张业新, 苏庆运, 王仲鹏, 高希彦, 张昭良.钾对镁铝水滑石复合氧化物的表面改性[J].物理化学学报, 2010, 26(4):921-926. doi: 10.3866/PKU.WHXB20100446ZHANG Ye-xin, SU Qing-yun, WANG Zhong-peng, GAO Xi-yan, ZHANG Zhao-liang. Surface modification of Mg-Al hydrotalcite mixed oxides with potassium[J]. Acta Phys-Chim Sin, 2010, 26(4):921-926. doi: 10.3866/PKU.WHXB20100446 [24] 王军利, 王虹, 孙志强, 任晓光. La-K-Co-Mn-O钙钛矿型复合氧化物同时去除碳颗粒和NOx的性能[J].环境污染与防治, 2008, 30(12):40-42. doi: 10.3969/j.issn.1001-3865.2008.12.010WANG Jun-li, WANG Hong, SUN Zhi-qiang, REN Xiao-guang. Performance of La-K-Co-Mn-O oxide catalysts for simultaneous removal of soot and NOx from diesel engine exhaust[J]. Environ Pollut Prevent, 2008, 30(12):40-42. doi: 10.3969/j.issn.1001-3865.2008.12.010 [25] FINO D, RUSSO N, SARACCO G, SPECCHIA V. Catalytic removal of NOx and diesel soot over nanostructured spinel-type oxides[J]. J Catal, 2006, 242(1):38-47. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=872897bbc02423e8f1bb9685dd6785dc [26] 王仲鹏, 陈铭夏, 上官文峰.类水滑石衍生CuAlO催化剂同时去除碳颗粒和氮氧化物[J].物理化学学报, 2009, 25(1):79-85. doi: 10.3866/PKU.WHXB20090114WANG Zhong-peng, CHEN Ming-xia, SHANG GUAN Wen-feng. Simultaneous catalytic removal of NOx and diesel soot over Cu-containing hydrotalcite derived catalysts[J]. Acta Phys-Chim Sin, 2009, 25(1):79-85. doi: 10.3866/PKU.WHXB20090114 [27] WANG Y, WEI HAN X, JI A, SHI L Y, HAYASHI S. Basicity of potassium-salt modified hydrotalcite studied by HMANMR using pyrrole as a probe molecule[J]. Microporous Mesoporous Mater, 2005, 77(2/3):139. http://www.sciencedirect.com/science/article/pii/S1387181104003427 [28] IORDAN A, ZAKI M I, KAPPENSTEIN C. Interfacial chemistry in the preparation of catalytic potassium-modified alumina[J]. J Chem Soc, 1993, 89(14):2527-2536. http://pubs.rsc.org/en/content/articlehtml/1993/ft/ft9938902527 [29] 刘鑫, 舒万艮, 桂客, 瞿龙.十二烷基苯磺酸柱撑类水滑石的热稳定性研究[J].中国塑料, 2004, 18(10):70-72. doi: 10.3321/j.issn:1001-9278.2004.10.016LIU Xin, SHU Wang-en, GUI Ke, QU Long. Study on heat stability of dodecyl benzene sulfonate pillared hydrotalcite[J]. China Plast, 2004, 18(10):70-72. doi: 10.3321/j.issn:1001-9278.2004.10.016 [30] TERAOKA Y, KANADA K, KAGAWA S. Synthesis of La-K-Mn-O perovskite-type oxides and their catalytic property for simultaneous removal of NOx and diesel soot particulates[J]. Appl Catal B:Environ, 2001, 34(1):73-78. doi: 10.1016/S0926-3373(01)00202-8 [31] NIU J R, DENG J G, LIU W, ZHANG L, WANG G Z, DAI H X, HE H, ZI X H. Nanosized perovskite-type oxides La1-xSrxMO3-δ (M=Co, Mn; x=0, 0.4) for the catalytic removal of ethylacetate[J]. Catal Today, 2007, 126:420-429. doi: 10.1016/j.cattod.2007.06.027 [32] KANNAN S, SWAMY C S. Catalytic decomposition of nitrous oxide over calcined cobalt aluminum hydrotalcites[J]. Catal Today, 1999, 53:725-737. doi: 10.1016/S0920-5861(99)00159-5 [33] HADNADJEV M, VUILC T, MARINKOVIC-NEDUCIN R, SUCHORSKI Y, WEISS H. The iron oxidation state in Mg-Al-Fe mixed oxides derived from layered double hydroxides:An XPS study[J]. Appl Surf Sci, 2008, 254:4297-4302. doi: 10.1016/j.apsusc.2008.01.063 -





 下载:
下载: