Impact of Ni content on the structure and adsorption desulfurization performance of Ni/ZnO-TiO2 adsorbent
-
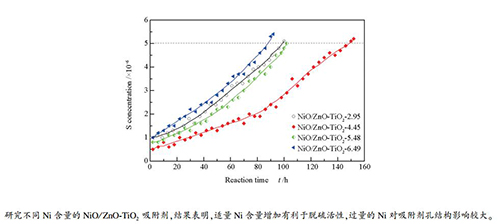
摘要: 以ZnO-TiO2为载体,采用等体积浸渍法制备了不同Ni含量的NiO/ZnO-TiO2汽油脱硫吸附剂。采用X射线衍射(XRD)、压汞、H2程序升温还原(H2-TPR)和H2程序升温脱附(H2-TPD)等手段对吸附剂进行了表征。同时,采用FCC轻汽油为原料,在固定床反应装置中对不同Ni含量的NiO/ZnO-TiO2吸附剂进行脱硫性能评价,以考察Ni含量对该吸附剂脱硫性能的影响。结果表明,Ni含量适量增加对于吸附剂比表面积、内部孔道分布和颗粒强度影响较小,同时能够增加具有脱硫活性的Ni0物种,促进吸附剂脱硫活性。当吸附剂中Ni质量分数达到5.48%后,吸附剂的内部孔道分布改变,吸附剂的比表面积和颗粒强度明显降低,对吸附剂脱硫活性极为不利。当Ni质量分数为4.45%时,吸附剂具有最佳脱硫性能,能够将FCC轻汽油中3×10-4的总硫含量降低至5×10-6以下,并维持脱硫时间达152 h,穿透硫容达11.24%(112.4 mg S/g吸附剂),且脱硫后FCC轻汽油烯烃含量变化较小。
-
关键词:
- FCC轻汽油 /
- 脱硫吸附 /
- Ni/ZnO-TiO2 /
- Ni含量
Abstract: Using ZnO-TiO2 as a carrier support, Ni/ZnO-TiO2 gasoline desulfurization adsorbents with different Ni contents were prepared by equal volume impregnation method and characterized by X-ray diffraction (XRD), Mercury intrusion porosimetry, H2-temperature-programmed desorption(H2-TPD) and H2-temperature-programmed reduction (H2-TPR). Meanwhile, the desulfurization performance of the Ni/ZnO-TiO2 adsorbents were evaluated using FCC light gasoline in a fixed bed reactor. The results show that proper increase of Ni content has little effect on the specific surface area, internal pore distribution and particle strength of the adsorbent, and can increase the Ni0 species with desulfurization activity and promote the desulfurization activity of the adsorbent. When the content of Ni in the adsorbent is too much, the internal pore distribution of the adsorbent changes, and the specific surface area and particle strength of the adsorbent are greatly reduced, which is extremely detrimental to the desulfurization activity of the adsorbent. When the Ni content is 4.45%, having the best desulfurization performance, can reduce the total sulfur content of 3×10-4 in FCC light gasoline to below 5×10-6, and maintain the desulfurization time up to 152 h, and the breakthrough sulfur capacity is 11.24% (112.4 mg S/g adsorbent). And the olefin content of FCC light gasoline after desulfurization changes little.-
Key words:
- FCC light gasoline /
- desulfurization adsorbent /
- Ni/ZnO-TiO2 /
- Ni content
-
表 1 NiO/ZnO-TiO2-x吸附剂的织构参数
Table 1 Textural properties of the NiO/ZnO-TiO2-x adsorbents
Absorbent ABET/
(m2·g-1)Pore volume v/
(mL·g-1)Average pore diameter
d/nmNiO/ZnO-TiO2-2.95 31.02 0.23 29.6 NiO/ZnO-TiO2-4.45 28.84 0.24 33.1 NiO/ZnO-TiO2-5.48 23.78 0.23 38.1 NiO/ZnO-TiO2-6.49 23.40 0.23 38.4 表 2 NiO/ZnO-TiO2-x吸附剂的颗粒强度
Table 2 Particle strength of the NiO/ZnO-TiO2-x adsorbents
Adsorbent NiO/ZnO-TiO2-2.95 NiO/ZnO-TiO2-4.45 NiO/ZnO-TiO2-5.48 NiO/ZnO-TiO2-6.49 Particle strength/(N·cm-1) 80.5 65.7 27.0 13.4 表 3 FCC轻汽油脱硫前后烯烃含量
Table 3 Olefins content of FCC gasoline before and after the desulfurization process
Sample Olefins
w/%Alkanes
w/%Density
ρ/(g·cm-3)FCC gasoline 42.6 55.27 0.6879 Ni/ZnO TiO2-2.95 43.2 54.71 0.6768 Ni/ZnO-TiO2-4.45 42.9 55.10 0.6796 Ni/ZnO-TiO2-5.48 42.3 55.96 0.6767 Ni/ZnO-TiO2-6.49 42.2 56.16 0.6842 -
[1] 钱伯章.清洁汽、柴油生产进程与技术进展(一)[J].润滑油与燃料, 2015, 25(5):1-5. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rhyyrl201505001QIAN Bo-zhang. Production process and technological progress of clean gas and diesel oil(Ⅰ)[J]. Lubes Fuels, 2015, 25(5):1-5. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rhyyrl201505001 [2] KHARE, GYANESH P, BARTLESVILL E. Desulfurization process and novel bimetallic sorbent systems for same: US, 6274533[P]. 2001-8-14. [3] HUANG L C, WANG G F, QIN Z F, DONG M, DU M X, GE H, LI X K, ZHAO Y D, ZHANG J, HU T D, WANG J G. In situ XAS study on the mechanism of reactive adsorption desulfurization of oil product over Ni/ZnO[J]. Appl Catal B:Environ, 2011, 106(1):26-38. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=af20580214ae4d0b757fd6c131606c93 [4] ZHAO L, CHEN Y, GAO J S, CHEN Y. Desulfurization mechanism of FCC gasoline:A review[J]. Front Chem Sci Eng, 2010, 4(3):314-321. doi: 10.1007/s11705-009-0271-9 [5] HUANG L X, WANG G F, QIN Z F, DU M X, DONG M, GE H, WU Z W, ZHAO Y D, MA C Y, HU T D, WANG J G. A sulfur K-edge XANES study on the transfer of sulfur species in the reactive adsorption desulfurization of diesel oil over Ni/ZnO[J]. Catal Commun, 2010, 11(7):592-596. doi: 10.1016/j.catcom.2010.01.001 [6] RYZHIKOV A, BEZVERKHYY I, BELLAT J P. Reactive adsorption of thiophene on Ni/ZnO:Role of hydrogen pretreatment and nature of the rate determining step[J]. Appl Catal B:Environ, 2008, 84(3):766-772. https://www.sciencedirect.com/science/article/pii/S0926337308002270 [7] PETZOLD F G, JASINSKI J, CLARK E L, KIM J H, ABSHER J, TOUFAR H, SUNKARA M K. Nickel supported on zinc oxide nanowires as advanced hydrodesulfurization catalysts[J]. Catal Today, 2012, 198(1):219-227. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=d57c947999cbb4c024544d40b1e995eb [8] ZHANG Y L, YANG Y X, HAN H X, YANG M, WANG L, ZHANG Y N, JANG Z X, LI C. Ultra-deep desulfurization via reactive adsorption on Ni/ZnO:The effect of ZnO particle size on the adsorption performance[J]. Appl Catal B:Environ, 2012, 119/120:13-19. doi: 10.1016/j.apcatb.2012.02.004 [9] HUSSAIN A H M S, TATARCHUK B J. Adsorptive desulfurization of jet and diesel fuels using Ag/TiOx-Al2O3 and Ag/TiOx-SiO2 adsorbents[J]. Fuel, 2013, 107(9):465-473. https://www.sciencedirect.com/science/article/pii/S0016236112009064 [10] RANA M S, MAITY S K, ANCHEYTA J, DHAR G M, RAO T S R P. TiO2-SiO2 supported hydrotreating catalysts:physico-chemical characterization and activities[J]. Appl Catal A:Gen, 2003, 253(1):165-176. doi: 10.1016/S0926-860X(03)00502-7 [11] STRANICK M A, HOUALLA M, HERCULES D M. The influence of TiO2 on the speciation and hydrogenation activity of Co/Al2O3catalysts[J]. J Catal, 1990, 125(1):214-226. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=10.1002/chin.199048021 [12] 邓存, 段连运, 徐献平, 谢有畅.复合载体TiO2/SiO2的气相吸附法制备及MoO3在其表面上的分散状态[J].催化学报, 1993, 14(4):281-286. http://www.cnki.com.cn/Article/CJFDTOTAL-CHUA199304005.htmDENG Cun, DUAN Lian-yun, XU Xian-ping, XIE You-chang. Preparation of composite carrier TiO2/SiO2 by gas adsorption and dispersion of MoO3 on its surface[J]. Chin J Catal, 1993, 14(4):281-286. http://www.cnki.com.cn/Article/CJFDTOTAL-CHUA199304005.htm [13] ROH H S, JUN K W, DONG W S, CHANG J S, PARK S E, JOE Y I. Highly active and stable Ni/Ce-ZrO2, catalyst for H2, production from methane[J]. J Mol Catal A:Chem, 2002, 181(1):137-142. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=91bdfd43b9ff7da1d1ab1862518d6ff5 [14] ULLAH R, BAI P, WU P P, ETIM U J, ZHANG J Q, HAN D Z, SUBHAN F, ULLAH S, ROOD M J, YAN Z F. Superior performance of freeze-dried Ni/ZnO-Al2O3 adsorbent in the ultra-deep desulfurization of high sulfur model gasoline[J]. Fuel Process Technol, 2016, 156:505-514. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=11d67dfa361024279a182b687f39eb97 [15] GOICOECHEA S, KRALEVA E, SOKOLOV S, SCHNEIDER M, POHI M M, KOCKMANN N, EHRICH H. Support effect on structure and performance of Co and Ni catalysts for steam reforming of acetic acid[J]. Appl Catal A:Gen, 2016, 514:182-191. doi: 10.1016/j.apcata.2015.12.025 [16] 熊峻, 陈吉祥, 张继炎.镍负载量对邻氯硝基苯加氢制邻氯苯胺Ni/TiO2催化剂性能的影响[J].催化学报, 2006, 27(7):579-584. doi: 10.3321/j.issn:0253-9837.2006.07.011XIONG Jun, CHEN Ji-xiang, ZHANG Ji-yan. Influence of Ni loading on properties of Ni/TiO2 catalyst for hydrogenation of o-chloronitrobenzene to o-chloroaniline[J]. Chin J Catal, 2006, 27(7):579-584. doi: 10.3321/j.issn:0253-9837.2006.07.011 [17] 唐明兴, 李学宽, 吕占军, 葛晖, 周立公.苯中硫在Ni/ZnO催化剂上加氢吸附脱除的研究[J].燃料化学学报, 2009, 37(6):707-712. doi: 10.3969/j.issn.0253-2409.2009.06.012TANG Ming-xing, LI Xue-kuan, LÜ Zhan-jun, GE Hui, ZHOU Li-gong. Ultra-deep hydrodesulfurization of benzene over Ni/ZnO catalyst[J]. J Fuel Chem Technol, 2009, 37(6):707-712. doi: 10.3969/j.issn.0253-2409.2009.06.012 [18] FERNANDES D M, SCOFIELD C F, NETO A A, CARDOSO M J B, ZOTIN J L, ZOTIN F M Z. The hydrogen adsorption capacity of commercial Pd/Rh and Pt/Rh automotive catalysts and its relationship to their activity[J]. Chem Eng J, 2012, 189/190:62-67. doi: 10.1016/j.cej.2012.02.024 [19] 杨霞, 田大勇, 孙守理, 孙琦. ZrO2-Al2O3复合载体对镍基催化剂甲烷化性能的影响[J].化工进展, 2014, 33(3):673-678. http://d.old.wanfangdata.com.cn/Periodical/hgjz201403030YANG Xia, TIAN Da-yong, SUN Shou-li, SUN Qi. Influence of zirconia-alumina composite on catalytic performance of nickel-based catalysts for methanation[J]. Chem Ind Eng Prog, 2014, 33(3):673-678. http://d.old.wanfangdata.com.cn/Periodical/hgjz201403030 [20] VELU S, GANGWAL S K. Synthesis of alumina supported nickel nanoparticle catalysts and evaluation of nickel metal dispersions by temperature programmed desorption[J]. Solid State Ionics, 2006, 177(7/8):803-811. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=e8750af8bb4bbeec708efaf8dbcf9880 [21] ISHIKAWA H, KONDO J N, DOMEN K. Hydrogen adsorption on Ru/ZrO2 studied by FT-IR[J]. J Phys Chem B, 1999, 103(16):3229-3234. doi: 10.1021/jp9842852 [22] WANG D H, QIAN W H, ISHIHARA A, KABE T. Elucidation of sulfidation state and hydrodesulfurization mechanism on TiO2 catalysts using 35S radioisotope tracer methods[J]. J Catal, 2001, 203(2):322-328. doi: 10.1006/jcat.2001.3349 -





 下载:
下载: