Experimental study on mercury removal of coal-fired flue gas over Co-doped iron-based oxide sorbent
-
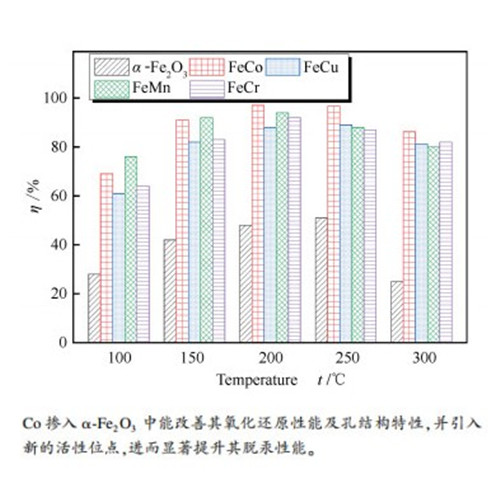
摘要: 使用柠檬酸法制备了Co掺杂的铁基氧化物(FeCo)吸附剂,通过固定床脱汞实验装置系统考察了FeCo吸附剂的脱汞性能,并利用比表面积(BET)、X射线衍射(XRD)、H2-程序升温还原(H2-TPR)、傅里叶红外光谱(FT-IR)、X射线光电子能谱(XPS)等表征手段分析吸附剂的物理化学特性。结果表明,α-Fe2O3中掺入Co后,比表面积、孔结构特性均得到改善,且氧化还原性能也相应提升;FeCo吸附剂在200-250℃获得最高约97%的脱汞效率;烟气中O2和NO的存在有助于FeCo吸附剂对Hg0的脱除,而SO2和H2O则抑制FeCo吸附剂对Hg0的脱除,同时NO能削弱SO2对FeCo脱汞的抑制作用。在脱汞过程中,FeCo吸附剂表面的活性组分Fe3+、Co3+和O*均消耗,参与了Hg0氧化反应,且吸附剂表面生成了HgO。在含SO2气氛中进行脱汞反应后,FeCo吸附剂表面发生硫酸盐化,从而削弱了吸附剂的脱汞性能,汞在吸附剂表面以HgO和HgSO4形式存在。Abstract: In this paper, the citric acid method was used to prepare the Co-doped iron-based oxide sorbent. The mercury removal performance of the FeCo sorbent was investigated by a fixed-bed mercury removal experimental device system, and the characterization methods of the specific surface area (BET), X-ray diffraction (XRD), H2-temperature programmed reduction (H2-TPR), Fourier infrared spectroscopy (FT-IR) and X-ray photoelectron spectroscopy (XPS) were performed to analyze the physical and chemical characteristics of the sorbent. The results of the study indicate that the specific surface area and pore structure characteristics are improved after the addition of Co into α -Fe2O3, and the redox performance of α -Fe2O3 is also improved. The maximum mercury removal efficiency of FeCo sorbent is obtained at 200-250 ℃ at the value of about 97%. The presence of O2 and NO in the gas benefits the removal of Hg0 over FeCo sorbent, while SO2 and H2O inhibit the removal of Hg0 over FeCo sorbent. The presence of NO can weaken the inhibitory effect of SO2 on mercury removal performance over FeCo. During the mercury removal process, the active components Fe3+, Co3+, and O* on the surface of the FeCo sorbent are consumed, particitate in the Hg0 oxidation process, and HgO is formed on the surface of the sorbent. After the mercury removal reaction in the atmosphere containing SO2, the sulfation of the sorbent surface is occurred, which weakens the mercury removal performance of the adsorbent.
-
Key words:
- mercury /
- iron /
- cobalt /
- coal-fired flue gas
-
表 1 样品的BET表征
Table 1 BET results of the samples
Sample ABET/(m2·g-1) vt/(cm3·g-1) dave/nm α-Fe2O3 24.57 0.19 26.52 FeCo 30.46 0.21 20.59 Co3O4 21.38 0.18 29.83 表 2 样品表面原子摩尔含量
Table 2 Mole content of the atoms on the surface of samples Samples
Sample Fe 2p /% Co 2p /% O 1s /% Fe2+/FeT Fe3+/FeT Co3+/CoT Co2+/CoT OL/OT O*/OT Fresh FeCo 37.2 62.8 47.2 52.8 50.7 49.3 FeCo-O 49.1 50.9 56.3 43.7 57.2 42.3 FeCo-S 41.3 58.7 51.6 48.4 53.4 46.2 FeT represents Fe2++Fe3+, CoT represents Co2++Co3+, OT represents OL +O* -
[1] YANG Y J, LIU J, WANG Z. Reaction mechanisms and chemical kinetics of mercury transformation during coal combustion[J]. Prog Energy Combust, 2020, 79:100844. doi: 10.1016/j.pecs.2020.100844 [2] 周强, 段钰锋, 卢平.燃煤电厂吸附剂喷射脱汞技术的研究进展[J].化工进展, 2018, 37(11):4460-4467. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hgjz201811044ZHOU Qiang, DUAN Yu-feng, LU Ping. Research progress on in-duct mercury removal by sorbent injection in power plant[J]. Chem Ind Eng Prog, 2018, 37(11):4460-4467. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hgjz201811044 [3] YANG J P, ZHANG M G, LI H L, QU W Q, ZHAO Y C, ZHANG J Y. Simultaneous NO reduction and Hg0 oxidation over La0.8Ce0.2MnO3 perovskite catalysts at low temperature[J]. Ind Eng Chem Res, 2018, 57(29):9374-9385. doi: 10.1021/acs.iecr.8b01431 [4] YANG Z Q, LI H L, YANG J P, FENG S H, LIU X, ZHAO J X, QU W Q, LI P, FENG Y, LEE P, SHIH K. Nanosized copper selenide functionalized zeolitic imidazolate framework-8(CuSe/ZIF-8) for efficient immobilization of gas-phase elemental mercury[J]. Adv Funct Mater, 2019, 29(17):1807191. doi: 10.1002/adfm.201807191 [5] ZHOU Q, LEI Y, LIU Y B, TAO X, LU P, DUAN Y F, WANG Y J. Gaseous elemental mercury removal by magnetic Fe-Mn-Ce sorbent in simulated flue gas[J]. Energy Fuels, 2018, 32(12):12780-12786. doi: 10.1021/acs.energyfuels.8b03445 [6] ZHANG S B, ZHAO Y C, YANG J P, ZHANG J Y, ZHENG C G. Fe-modified MnOx/TiO2 as the SCR catalyst for simultaneous removal of NO and mercury from coal combustion flue gas[J]. Chem Eng J, 2018, 348:618-629. doi: 10.1016/j.cej.2018.05.037 [7] LIU Y, WANG Y J, WANG H Q, WU Z B. Catalytic oxidation of gas-phase mercury over Co/TiO2 catalysts prepared by sol-gel method[J]. Catal Commun, 2011, 12(14):1291-1294. doi: 10.1016/j.catcom.2011.04.017 [8] CHEN G Y, ZHANG D, ZHANG A C, ZHANG Z H, LIU Z C, HOU L. CrOx-MnOx-TiO2 adsorbent with high resistance to SO2 poisoning for Hg0 removal at low temperature[J]. J Ind Eng Chem, 2017, 55:119-127. doi: 10.1016/j.jiec.2017.06.035 [9] ZHAO L K, LI C T, DU X Y, ZENG G M, GAO L, ZHAI Y B, WANG T, ZHANG J Y. Effect of Co addition on the performance and structure of V/ZrCe catalyst for simultaneous removal of NO and Hg0 in simulated flue gas[J]. Appl Surf Sci, 2018, 437:390-399. doi: 10.1016/j.apsusc.2017.08.165 [10] HUANG W J, XU H M, QU Z, ZHAO S J, CHEN W M, YAN N Q. Significance of Fe2O3 modified SCR catalyst for gas-phase elemental mercury oxidation in coal-fired flue gas[J]. Fuel Process Technol, 2016, 149:23-28. doi: 10.1016/j.fuproc.2016.04.007 [11] BAUER I, KNoLKER H-J. Iron catalysis in organic synthesis[J]. Chem Rev, 2015, 115(9):3170-3387. doi: 10.1021/cr500425u [12] CHEN C, ZUO W Q, YANG J C, CUI H J, FU M L. Yolk-shell structured CoFe2O4 microspheres as novel catalysts for peroxymonosulfate activation for efficient degradation of butyl paraben[J]. RSC Adv, 2016, 6(103):101361-101364. doi: 10.1039/C6RA24101H [13] LIU T, MAN C Y, GUO X, ZHENG C G. Experimental study on the mechanism of mercury removal with Fe2O3 in the presence of halogens:Role of HCl and HBr[J]. Fuel, 2016, 173:209-216. doi: 10.1016/j.fuel.2016.01.054 [14] MENG B, ZHAO Z B, WANG X Z, LIANG J J, QIU J S. Selective catalytic reduction of nitrogen oxides by ammonia over Co3O4 nanocrystals with different shapes[J]. Appl Catal B:Environ, 2013, 129:491-500. doi: 10.1016/j.apcatb.2012.09.040 [15] GAO L, LI C T, LI S H, ZHANG W, DU X Y, HUANG L, ZHU Y C, ZHAI Y B, ZENG G M. Superior performance and resistance to SO2 and H2O over CoOx-modified MnOx/biomass activated carbons for simultaneous Hg0 and NO removal[J]. Chem Eng J, 2019, 371:781-795. doi: 10.1016/j.cej.2019.04.104 [16] ZHANG A C, ZHENG W W, SONG J, HU S, LIU ZX, XIANG J. Cobalt manganese oxides modified titania catalysts for oxidation of elemental mercury at low flue gas temperature[J]. Chem Eng J, 2014, 236:29-38. doi: 10.1016/j.cej.2013.09.060 [17] ZHANG X P, CUI Y Z, WANG J X, TAN B J, LI C F, ZHANG H, HE G H. Simultaneous removal of Hg0 and NO from flue gas by Co0.3-Ce0.35-Zr0.35O2 impregnated with MnOx[J]. Chem Eng J, 2017, 326:1210-1222. doi: 10.1016/j.cej.2017.06.014 [18] SHI Y J, DENG S, WANG H M, HUANG J Y, LI Y K, ZHANG F, SHU X Q. Fe and Co modified vanadium-titanium steel slag as sorbents for elemental mercury adsorption[J]. RSC Adv, 2016, 6(19):15999-16009. doi: 10.1039/C5RA26712A [19] SHAO C Z, LIU X F, MENG D M, XU Q, GUO Y L, GUO Y, ZHAN W C, WANG L, LU G Z. Catalytic performance of Co-Fe mixed oxide for NH3-SCR reaction and the promotional role of cobalt[J]. RSC Adv, 2016, 6(70):66169-66179. doi: 10.1039/C6RA12025C [20] LI H H, WANG S K, WANG X, HU J J. Activity of CuCl2-modified cobalt catalyst supported on Ti-Ce composite for simultaneous catalytic oxidation of Hg0 and NO in a simulated pre-sco process[J]. Chem Eng J, 2017, 316:1103-1113. doi: 10.1016/j.cej.2017.02.052 [21] ZHANG P, PAN W G, GUO R T, ZHU X B, LIU J, QIN L, SHE X L. The Mo modified Ce/TiO2 catalyst for simultaneous Hg0 oxidation and NO reduction[J]. J Energy Inst, 2019, 92(5):1313-1328. doi: 10.1016/j.joei.2018.10.003 [22] APOSTOLESCU N, GEIGER B, HIZBULLAH K, JAN M T, KURETI S, REICHERT D, SCHOTT F, WEISWEILER W. Selective catalytic reduction of nitrogen oxides by ammonia on iron oxide catalysts[J]. Appl Catal B:Environ, 2006, 62(1):104-114. http://www.sciencedirect.com/science/article/pii/S0926337305002857 [23] LIU C X, GONG L, DAI R Y, LU M J, SUN T T, LIU Q, HUANG X G, HUANG Z. Mesoporous Mn promoted Co3O4 oxides as an efficient and stable catalyst for low temperature oxidation of CO[J]. Solid State Sci, 2017, 71:69-74. doi: 10.1016/j.solidstatesciences.2017.07.006 [24] LI H H, WANG S K, WANG X, TANG N, PAN S W, HU J J. Comparative study of Co/TiO2, Co-Mn/TiO2 and Co-Mn/Ti-Ce catalysts for oxidation of elemental mercury in flue gas[J]. Chem Pap, 2017, 71(9):1569-1578. doi: 10.1007/s11696-017-0152-5 [25] CHEN W, ZHANG Z A, BAO W Z, LAI Y Q, LI J, GAN Y Q, WANG J J. Hierarchical mesoporous γ-Fe2O3/carbon nanocomposites derived from metal organic frameworks as a cathode electrocatalyst for rechargeable Li-O2 batteries[J]. Electrochim Acta, 2014, 134:293-301. doi: 10.1016/j.electacta.2014.04.110 [26] SMIRNIOTIS P G, SREEKANTH P M, PEND A, JENKINS R G. Manganese oxide catalysts supported on TiO2, Al2O3, and SiO2:A Comparison for Low-Temperature SCR of NO with NH3[J]. Ind Eng Chem Res, 2006, 45(19):6436-6443. doi: 10.1021/ie060484t [27] DONG L, HUANG Y J, CHEN H, LIU L Q, LIU C Q, XU L G, ZHA J R, WANG Y X, LIU H. Magnetic γ-Fe2O3-loaded attapulgite sorbent for Hg0 removal in coal-fired flue gas[J]. Energy Fuels, 2019, 33(8):7522-7533. doi: 10.1021/acs.energyfuels.9b01136 [28] WU H Y, LI C T, ZHAO L K, ZHANG J, ZENG G M, XIE Y N, ZHANG X N, WANG Y. Removal of gaseous elemental mercury by cylindrical activated coke loaded with CoOx-CeO2 from simulated coal combustion flue gas[J]. Energy Fuels, 2015, 29(10):6747-6757. doi: 10.1021/acs.energyfuels.5b00871 [29] LIU D J, ZHOU W G, WU J. Effect of Ce and La on the activity of CuO/ZSM-5 and MnOx/ZSM-5 composites for elemental mercury removal at low temperature[J]. Fuel, 2017, 194:115-122. doi: 10.1016/j.fuel.2016.12.076 [30] 于贤群, 鲍静静, 姜小祥, 杨宏旻. Mn-TiO2催化剂烟气脱汞性能及反应机理[J].中国电机工程学报, 2015, 35(13):3331-3337. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgdjgcxb201513016YU Xian-qun, BAO Jing-jing, JIANG Xiao-xiang, YANG Hong-min. Performance and mechanism of catalytic oxidation for mercury by Mn-doped TiO2 catalysts in flue gas[J]. Proc CSEE, 2015, 35(13):3331-3337. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgdjgcxb201513016 [31] ZHANG D, HOU L, CHEN G Y, ZHANG A C, WANG F H, WANG R R, LI C W. Cr Doping MnOx adsorbent significantly improving Hg0 removal and SO2 resistance from coal-fired flue gas and the mechanism investigation[J]. Ind Eng Chem Res, 2018, 57(50):17245-17258. doi: 10.1021/acs.iecr.8b04857 [32] YANG W, LIU Y X, WANG, PAN J F. Removal of elemental mercury from flue gas using wheat straw chars modified by Mn-Ce mixed oxides with ultrasonic-assisted impregnation[J]. Chem Eng J, 2017, 326:169-181. doi: 10.1016/j.cej.2017.05.106 [33] SHAN Y, YANG W, LI Y, LIU Y X, PAN J F. Preparation of microwave-activated magnetic bio-char adsorbent and study on removal of elemental mercury from flue gas[J]. Sci Total Environ, 2019, 697:134049. doi: 10.1016/j.scitotenv.2019.134049 [34] ZHANG X P, LI Z F, WANG J X, TAN B J, CUI Y Z, HE G H. Reaction mechanism for the influence of SO2 on Hg0 adsorption and oxidation with Ce0.1-Zr-MnO2[J]. Fuel, 2017, 203:308-315. doi: 10.1016/j.fuel.2017.04.065 [35] WAN Q, DUAN L, HE K B, LI J H. Removal of gaseous elemental mercury over a CeO2-WO3/TiO2 nanocomposite in simulated coal-fired flue gas[J]. Chem Eng J, 2011, 170(2):512-517. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=b15df7cc054faf86a82ac352c510127b [36] LI Y, MURPHY P D, WU C Y, POWERS K, BONZONGO J J. Development of silica/vanadia/titania catalysts for removal of elemental mercury from coal-combustion flue gas[J]. Environ Sci Technol, 2008, 42(14):5304-5309. doi: 10.1021/es8000272 [37] YANG S J, GUO Y F, YAN N Q, WU D Q, HE H P, XIE J K, QU Z, JIA J P. Remarkable effect of the incorporation of titanium on the catalytic activity and SO2 poisoning resistance of magnetic Mn-Fe spinel for elemental mercury capture[J]. Appl Catal B:Environ, 2011, 101(3):698-708. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=495c63f00ef74aa3031a167b713ddd3e [38] GAO L, LI C T, ZHANG J, DU X Y, LI S H, ZENG J W, YI Y Y, ZENG G M. Simultaneous removal of NO and Hg0 from simulated flue gas over CoOx-CeO2 loaded biomass activated carbon derived from maize straw at low temperatures[J]. Chem Eng J, 2018, 342:339-349. doi: 10.1016/j.cej.2018.02.100 [39] SHEN B X, ZHU S W, ZHANG X, CHI G L, PATEL D, SI M, WU C F. Simultaneous removal of NO and Hg0 using Fe and Co co-doped Mn-Ce/TiO2 catalysts[J]. Fuel, 2018, 224:241-249. doi: 10.1016/j.fuel.2018.03.080 [40] ZHANG A C, ZHANG Z H, LU H, LIU Z C, XIANG J, ZHOU C S, XING W B, SUN L S. Effect of promotion with Ru addition on the activity and SO2 resistance of MnOx-TiO2 adsorbent for Hg0 removal[J]. Ind Eng Chem Res, 2015, 5(11):2930-2939. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=0147f4d8ac1da94d94c34490f8544b24 -





 下载:
下载: