Effect of ZSM-5 crystal size on its catalytic properties for conversion of methanol to gasoline
-
摘要: 通过调控水热合成ZSM-5凝胶液中H2O/Si物质的量比,实现了粒径为70、200、400和650 nm四种单分散ZSM-5的可控合成。采用XRD、TEM、BET和NH3-TPD等多种表征对其微观结构进行分析,结合催化性能评价,考察了晶粒粒径对其催化甲醇制汽油反应性能的影响机制。结果表明,整体上随着ZSM-5晶粒粒径的增加,其外比表面积减小,结晶度提高,酸量呈现出先增加后基本不变的趋势。但外表面附着小晶粒的粒径为650 nm的分子筛体现出了大的外表面积和强的酸性。ZSM-5晶粒粒径的增加整体上降低了其催化MTG反应的寿命和最高收率。晶粒粒径为70 nm时,ZSM-5体现出了96 h的催化寿命和30.8%的最高收率。晶粒粒径为650 nm样品由于其大的外比表面积和较强的表面酸性,也体现出91 h的寿命。在大晶粒ZSM-5外表面附着生长小晶粒ZSM-5,是一种制备高性能催化剂的新方法。Abstract: Four-sized monodispersed ZSM-5 crystals, being 70, 200, 400 and 650 nm, were hydrothermally synthesized by changing the H2O/Si molar ratio in the synthesis gel, and characterized with XRD, TEM, BET and NH3-TPD techniques. The crystal size effect of ZSM-5 on its catalytic performance for conversion of methanol to gasoline (MTG) was investigated. It was shown that the external surface area of the sample decreased with its crystal size, while the acid site amount firstly increased, and then kept almost constant. Nevertheless, 650 nm ZSM-5 crystals attaching small particles exhibit large external surface area and strong acidity. The catalytic stability and the liquid hydrocarbon yield decreased with increasing crystal size. The sample with a crystal size of 70 nm shows a catalytic lifetime of 96 h and a gasoline yield of 30.8%. The large external surface area and relatively strong acidity endow the sample with a crystal size of 650 nm also has a catalytic lifetime of 91 h, indicating that synthesis of large ZSM-5 crystals with small crystallites adhered to their surface could be a potential way to improve the catalytic performance.
-
Key words:
- ZSM-5 /
- H2O/Si molar ratio /
- crystal size effect /
- diffusion property /
- MTG catalytic performance
-
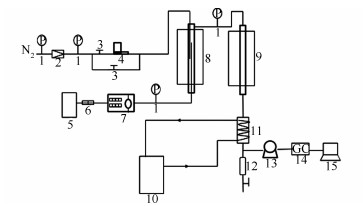
图 1 固定床催化剂评价装置示意图
Figure 1 Diagram of MTG reaction set-up
1: pressure gauge; 2: pressure reducing value; 3: globe value; 4: gas flowmeter; 5: stock tank; 6: filter; 7: micro tube pump; 8: preheater; 9: reactor; 10: condensate recirculating tank; 11: condensator; 12: liquid storage tank; 13: wet gas flowmeter; 14: gas chromatography (Agilent GC); 15: computer
表 1 不同催化剂的相对结晶度
Table 1 Relative crystallinity of different-sized ZSM-5 samples
Catalyst ZY5-70 ZY5-200 ZY5-400 ZY5-650 Relative crystallinitya/% 56 63 70 100 a: the crystallinity of samples was calculated by comparing the intensity of diffraction peaks between 20.0° and 25.0° with that of ZY5-650 表 2 不同催化剂的结构性质
Table 2 Textural properties of different-sized ZSM-5 samples
Catalyst Crystal size d/nm ABETa/(m2·g-1) Aextb/(m2·g-1) vmicrob/(cm3·g-1) vmesoc/(cm3·g-1) ZY5-70 70 401 136 0.12 0.88 ZY5-200 200 401 69 0.16 0.31 ZY5-400 400 421 58 0.16 0.22 ZY5-650 650 397 62 0.17 0.23 a: derived from the BET model, b: by t-plod method, c: using the BJH method by desorption, Aext: mesopore surface area, vmicro: micropore volume, vmeso: mesopore volume 表 3 不同催化剂的酸性质
Table 3 Acid site amount of different-sized ZSM-5 samples
Sample Distribution of acid sites /(mmol·g-1) total weak (120-250 ℃) medium (250-350 ℃) strong (350-550 ℃) ZY5-70 0.20 0.04 0.11 0.05 ZY5-200 0.50 0.15 0.10 0.25 ZY5-400 0.47 0.15 0.08 0.24 ZY5-650 0.49 0.12 0.08 0.29 表 4 不同催化剂的活性
Table 4 Catalytic results of different-sized ZSM-5 samples
Sample Liquid hydrocarbon Catalyst lifetime t/h productiona/(g·gzeolite) yield at steady stage w/% ZY5-70 102 30.8 96 ZY5-200 85 28.8 86 ZY5-400 67 27.6 78 ZY5-650 92 29.8 91 -
[1] 胡津仙, 胡靖文, 王俊杰, 相宏伟, 李永旺.甲醇在不同酸性ZSM-5上转化为汽油(MTG)的研究[J].天然气化工, 2001, 26(6):12-15. http://www.cnki.com.cn/Article/CJFDTOTAL-TRQH200106000.htmHU Jin-xian, HU Jing-wen, WANG Jun-jie, XIANG Hong-wei, LI Yong-wang. Study of MTG process with ZSM-5 zeolites which have different acid properties[J]. Nat Gas Chem Ind, 2001, 26(6):12-15. http://www.cnki.com.cn/Article/CJFDTOTAL-TRQH200106000.htm [2] 钟炳, 罗庆云, 肖有燮, 张威.甲醇在HZSM-5上转化为烃类的催化反应机理[J].燃料化学学报, 1986, 14(1):9-16. http://www.cnki.com.cn/Article/CJFDTOTAL-RLHX198601001.htmZHONG Bing, LUO Qing-yun, XIAO You-xie, ZHANG Wei. Reaction mechanism of mehtnaol to hydrocatbons on HZSM-5[J]. J Fuel Chem Technol, 1986, 14(1):9-16. http://www.cnki.com.cn/Article/CJFDTOTAL-RLHX198601001.htm [3] TIAN P, WEI Y X, YE M, LIU Z M. Methanol to olefins (MTO):From fundamentals to commercialization[J]. ACS Catal, 2015, 5(3):1922-1938. doi: 10.1021/acscatal.5b00007 [4] XIE Z K, LIU Z C, WANG Y D, JIN Z H. Applied catalysis for sustainable development of chemical industry in China[J].Nat Sci Rev, 2015, 2(2):167-182. doi: 10.1093/nsr/nwv019 [5] MEI C S, WEN P Y, LIU Z C, LIU H X, WANG Y D, YANG W M, XIE Z K, HUA W M, GAO Z. Selective production of propylene from methanol:Mesoporosity development in high silica HZSM-5[J]. J Catal, 2008, 258(1):243-249. doi: 10.1016/j.jcat.2008.06.019 [6] BJØRGEN M, JOENSEN F, SPANGSBERG H M, OLSBYE U, LILLERYD K P, SVELLE S. Methanol to gasoline over zeolite H-ZSM-5:Improved catalyst performance by treatment with NaOH[J]. Appl Catal A:Gen, 2008, 345(1):43-50. doi: 10.1016/j.apcata.2008.04.020 [7] 苗青, 董梅, 牛宪军, 王浩, 樊卫斌, 王建国, 秦张峰.含镓ZSM-5分子筛的制备及其在甲醇芳构化反应中的催化性能[J].燃料化学学报, 2012, 40(10):1230-1239. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18048.shtmlMIAO Qing, DONG Mei, NIU Xian-jun, WANG Hao, FAN Wei-bin, Wang Jian-guo, QIN Zhang-feng. Synthesis of gallium-containing ZSM-5 molecular sieves and their catalytic performance in methanol aromatization[J]. J Fuel Chem Technol, 2012, 40(10):1230-1239. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18048.shtml [8] 许烽, 董梅, 苟蔚勇, 黄立志, 李俊汾, 樊卫斌, 秦张峰, 王建国. ZSM-5分子筛的粒径可控合成及其在甲醇转化中的催化作用[J].燃料化学学报, 2012, 40(5):576-582. http://rlhxxb.sxicc.ac.cn/CN/Y2012/V40/I05/576XU Feng, DONG Mei, GOU Wei-yong, HUANG Li-zhi, LI Jun-fen, FAN Wei-bin, QIN Zhang-feng, WANG Jian-guo. Size-controllable synthesis of ZSM-5molecular sieves and their catalytic performance in the conversion of methanol to hydrocarbons[J]. J Fuel Chem Technol, 2012, 40(5):576-582. http://rlhxxb.sxicc.ac.cn/CN/Y2012/V40/I05/576 [9] CORMA A. From microporous to mesoporous molecular sieve materials and their use in catalysis[J]. Chem Rev, 1997, 97(6):2373-2420. doi: 10.1021/cr960406n [10] 常江伟, 付廷俊, 李忠. ZSM-5晶粒尺寸调控及其催化甲醇制烃过程积炭的形成及落位研讨[J].天然气化工(C1化学与化工), 2016, 41(1):61-67. http://www.cnki.com.cn/Article/CJFDTOTAL-TRQH201601022.htmCHANG Jiang-wei, FU Ting-jun, LI Zhong. The study of size control of ZSM-5 crystal and the coke formation and location on ZSM-5 during methanol to hydrocarbons conversion process[J]. Nat Gas Chem Ind, 2016, 41(1):61-67. http://www.cnki.com.cn/Article/CJFDTOTAL-TRQH201601022.htm [11] BLEKEN F L, BARBERA K, BONINO F, OLSBYE U, LILLERUD K P, BORDIGA S, BEATO P, JANSSENS T V W, SVELLE S. Catalyst deactivation by coke formation in microporous and desilicated zeolite H-ZSM-5 during the conversion of methanol to hydrocarbons[J]. J Catal, 2013, 307(6):62-73. http://www.academia.edu/4659371/Catalyst_deactivation_by_coke_formation_in_microporous_and_desilicated_zeolite_H-ZSM-5_during_the_conversion_of_methanol_to_hydrocarbons [12] 温鹏宇, 梅长松, 刘红星, 杨为民, 陈庆龄.甲醇制丙烯过程中ZSM-5催化剂的失活行为[J].石油学报(石油加工), 2008, 24(4):446-450. http://www.cnki.com.cn/Article/CJFDTOTAL-SXJG200804017.htmWEN Peng-yu, MEI Chang-song, LIU Hong-xing, YANG Wei-min, CHEN Qing-ling. Deactivation of zsm-5 catalysts during methanol-to-propylene process[J]. Acta Pet Sin (Pet Process), 2008, 24(4):446-450. http://www.cnki.com.cn/Article/CJFDTOTAL-SXJG200804017.htm [13] MCLELLAN G D, HOWE R F, PARKER L M, BIBBY D M. Effects of coke formation on the acidity of ZSM-5[J]. J Catal, 1986, 99(2):486-491. doi: 10.1016/0021-9517(86)90373-8 [14] LEE J, HONG U G, HWANG S, YOUN M H, SONG I K. Production of light olefins through catalytic cracking of C5 raffinate over carbon-templated ZSM-5[J]. Fuel Process Technol, 2013, 108(4):25-30. https://www.researchgate.net/publication/257210492_Production_of_light_olefins_through_catalytic_cracking_of_C5_raffinate_over_carbon-templated_ZSM-5?_sg=D_deiv5IjNftvocqHQ2KoEYDT6oAlVLOLIpp3HY7Vnsvdl0hMhHYJmk2NFwtsAsswnEVkjkCg13vrylnrrxdRg [15] HU Z, ZHANG H B, WANG L, ZHANG H X, ZHANG Y H, XU H L, SHEN W, TANG Y. Highly stable boron-modified hierarchical nanocrystalline ZSM-5 zeolite for the methanol to propylene reaction[J]. Catal Sci Technol, 2014, 4(9):2891-2895. doi: 10.1039/C4CY00376D [16] XIAO Q, YAO Q S, ZHUANG J, LIU G, ZHONG Y J, ZHU W D. A localized crystallization to hierarchical ZSM-5 microspheres aided by silane coupling agent[J]. J Colloid Interface Sci, 2013, 394(1):604-610. https://www.researchgate.net/publication/234141667_A_localized_crystallization_to_hierarchical_ZSM-5_microspheres_aided_by_silane_coupling_agent [17] MOCHIZUKI H, YOKOI T, IMAI H, WATANABE R, NAMBA S, KONDO J N, TATSUMI T. Facile control of crystallite size of ZSM-5 catalyst for cracking of hexane[J]. Microporous Mesoporous Mater, 2011, 145(1/3):165-171. https://www.researchgate.net/publication/251673944_Facile_control_of_crystallite_size_of_ZSM-5_catalyst_for_cracking_of_hexane [18] TAO Y S, KANOH H, ARAMS L, KANEKO K. Mesopore-modified zeolites:Preparation, characterization, and applications[J]. Chem Rev, 2006, 106(3):896-910. doi: 10.1021/cr040204o [19] 卢信清, 许春慧, 张富民, 钟依均, 朱伟东.碱处理制备介孔-微孔沸石分子筛的影响因素及其应用研究进展[J].化工进展, 2014, 33(8):2038-2043. http://www.cnki.com.cn/Article/CJFDTOTAL-HGJZ201408018.htmLU Xin-qing, XU Chun-hui, ZHANG Fu-min, ZHONG Yi-jun, ZHU Wei-dong. Influence factors for preparation of meso-microporous zeolites by alkali-treatment and their research progress[J].Chem Ind Eng Prog, 2014, 33(8):2038-2043. http://www.cnki.com.cn/Article/CJFDTOTAL-HGJZ201408018.htm [20] KORTUNOV P, VASENKOV S, KÄRGER J, VALIULLIN R, GOTTSCHALK P, FÉ ELÍAM, PEREZ M, STÓCKER M, DRESCHER B, MCELHINEY G, BERGER C, GLÄSER R, WEITKAMP J. The role of mesopores in intracrystalline transport in USY zeolite:PFG NMR diffusion study on various length scales[J]. J Am Chem Soc, 2005, 127(37):13055-13059. doi: 10.1021/ja053134r [21] ABELLÓS, BONILLA A, PÉREZ-RAMÍREZ J. Mesoporous ZSM-5 zeolite catalysts prepared by desilication with organic hydroxides and comparison with NaOH leaching[J]. Appl Catal A:Gen, 2009, 364(1/2):191-198. [22] OGURA M, SHINOMIYA S Y, TATENOA J, NARA Y, NOMURA M, KIKUCHI E, MATSULATA M. Alkali-treatment technique-new method for modification of structural and acid-catalytic properties of ZSM-5 zeolites[J]. Appl Catal A:Gen, 2001, 219(1/2):33-43. https://www.researchgate.net/publication/222877270_Alkali-treatment_technique_-_New_method_for_modification_of_structural_and_acid-catalytic_properties_of_ZSM-5_zeolites [23] 常江伟, 付廷俊, 张洪建, 周浩, 李忠.碱处理浓度对预脱铝ZSM-5成孔过程及其MTG反应性能的影响[J].无机化学学报, 2015, 31(11):2119-2127. http://www.cnki.com.cn/Article/CJFDTOTAL-WJHX201511004.htmCHANG Jiang-wei, FU Ting-jun, ZHANG Hong-jian, ZHOU Hao, LI Zhong. Effect of alkaline concentration on mesopore formation in acid pre-treated HZSM-5 zeolite and its catalytic performance in the methanol-to-gasoline reaction[J]. Chin J Inorg Chem, 2015, 31(11):2119-2127. http://www.cnki.com.cn/Article/CJFDTOTAL-WJHX201511004.htm [24] MAJANO G, DARWICHE A, MINTOVA S, VALTCHEV V. Seed-induced crystallization of nanosized na-ZSM-5 crystals[J]. Ind Eng Chem Res, 2009, 48(15):7084-7091. doi: 10.1021/ie8017252 [25] TOSHEVA L, VALTCHEV V P. Nanozeolites:Synthesis, crystallization mechanism, and applications[J]. Chem Mater, 2005, 17(10):2494-2513. doi: 10.1021/cm047908z [26] ALIPOUR S M. Recent advances in naphtha catalytic cracking by nano ZSM-5:A review[J]. Chin J Catal, 2016, 37(5):671-680. doi: 10.1016/S1872-2067(15)61091-9 [27] REDDY J K, MOTOKURA K, KOYAMA T R, MIYAJI A, BABA T. Effect of morphology and particle size of ZSM-5 on catalytic performance for ethylene conversion and heptane cracking[J]. J Catal, 2012, 289(5):53-61. https://www.researchgate.net/publication/256737932_Effect_of_morphology_and_particle_size_of_ZSM-5_on_catalytic_performance_for_ethylene_conversion_and_heptane_cracking [28] ROWNAGHI A A, HEDLUND J. Methanol to gasoline-range hydrocarbons:Influence of nanocrystal size and mesoporosity on catalytic performance and product distribution of ZSM-5[J]. Ind Eng Chem Res, 2011, 50(21):11872-11878. doi: 10.1021/ie201549j [29] SHIRALKAR V P, JOSHI P N, EAPEN M J, RAO B S. Synthesis of ZSM-5 with variable crystallite size and its influence on physicochemical properties[J]. Zeolites, 1991, 11(5):511-516. doi: 10.1016/S0144-2449(05)80127-7 [30] ZHANG H B, MA Y C, SONG K S, ZHANG Y H, TANG Y. Nano-crystallite oriented self-assembled ZSM-5 zeolite and its LDPE cracking properties:Effects of accessibility and strength of acid sites[J]. J Catal, 2014, 302(6):115-125. https://www.researchgate.net/profile/Hongbin_Zhang5/publication/256738000_Corrigendum_to_Nano-crystallite_oriented_self-assembled_ZSM-5_zeolite_and_its_LDPE_cracking_properties_Effects_of_accessibility_and_strength_of_acid_sites_J_Catal_302_2013_115-125/links/00b7d52e31fbaf330a000000.pdf [31] FIROOZI M, BAGHALHA M, ASADI M. The effect of micro and nano particle sizes of H-ZSM-5 on the selectivity of MTP reaction[J]. Catal Commun, 2009, 10(12):1582-1585. doi: 10.1016/j.catcom.2009.04.021 [32] LARSEN S C. Nanocrystalline zeolites and zeolite structures:Synthesis, characterization, and applications[J]. J Phys Chem C, 2007, 111(50):18464-18474. doi: 10.1021/jp074980m [33] SONG W, JUSTICE R E, JONES C A, GRASSIAN V H, LARSEN S C. Size-dependent properties of nanocrystalline silicalite synthesized with systematically varied crystal sizes[J]. Langmuir, 2004, 20(11):4696-4702. doi: 10.1021/la049817m -





 下载:
下载: