-
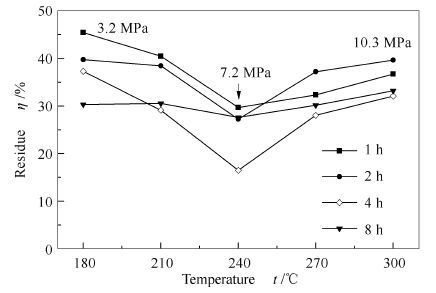
摘要: 采用微型高温高压反应釜,在超/亚临界乙醇体系,进行麦草碱木质素的解聚实验,通过扫描电子显微镜(SEM)、气相色谱/质谱联用仪(GC/MS)及红外光谱仪(FT-IR)对解聚产物进行分析,探讨大分子结构的解聚机理。结果表明,碱木质素在乙醇临界点条件(240℃,7.2 MPa)解聚获得最低残焦得率,数值为16.5%。碱木质素在亚临界乙醇体系解聚过程,碱木质素熔融形成直径1.0-2.0 μm的微球分散于乙醇中,结构单体间少量醚键和苯环侧链Cα均裂断裂,形成酚类、酯类、酮类和酸类产物;碱木质素在超临界乙醇体系解聚过程,熔融微球直径明显缩小,解聚时发生大量结构单体间醚键、苯环侧链Cα断裂及酯类产物的二次分解反应,解聚产物中酯类产物含量(11.94%)降低,酚类产物得率(52.14%)提高。Abstract: The depolymerization process of wheat straw alkali lignin in sub- and supercritical ethanol was investigated with a micro autoclave reactor. The degraded product properties and the depolymerization mechanism of lignin structure were studied by scanning electron microscopy (SEM), gas chromatography/mass spectrometry (GC/MS) and infrared spectroscopy (FT-IR). The experimental results show that the minimum residual char yield (16.5%) is obtained at the condition of ethanol supercritical point (240℃, 7.2 MPa). Under subcritical ethanol conditions, alkali lignin firstly melts and disperses in ethanol as 1.0-2.0 μm diameter of microspheres, then a small amount of ether linkages and benzene ring side chain Cα are broken to form phenols, esters, ketones and acids products. Under supercritical ethanol conditions, the diameter of molten microsphere is significantly reduced, and plenty of ether linkages and benzene ring side chain Cα are continuously broken, meanwhile, the lipid products are subjected to secondary decomposition reaction. The yield of lipid is decreased (11.94%), while the yield of phenolic products from depolymerization is increased (52.14%).
-
Key words:
- alkali lignin /
- sub- and supercritical ethanol /
- depolymerization
-
表 1 实验原料的工业分析和元素分析
Table 1 Ultimate and proximate analyses of the materials
Materials Proximate analysis w/% Ultimate analysis w/% Na/10-6 A V FC C H O* N Alkali lignin 0.22 54.18 45.60 56.01 8.54 35.34 0.08 - *: by difference 表 2 碱木质素超/亚临界乙醇解聚液相产物组成的GC/MS分析
Table 2 Oil GC/MS of alkali lignin depolymerization in sub-and supercritical ethanol
Retention time t/min Products name Molecular formula Content w/% 180 ℃ 210 ℃ 240 ℃ 270 ℃ 300 ℃ 12.41 phenol 
- 2.89 2.97 0.46 5.65 15.47 phenol, 2-methoxy- - 8.54 10.77 13.06 12.82 18.32 phenol, 4-ethyl- - - 1.77 2.79 6.63 18.62 1, 4-benzenediol, 2, 5-dimethyl- - - - - 3.41 18.74 2-methoxy-4-methylphenol - 3.77 5.74 7.45 8.22 20.54 1, 2-benzenediol, 3-methoxy- - - 4.17 - - 20.98 phenol, 2-ethyl-4-methyl- - - - - 2.26 21.23 phenol, 4-ethyl-2-methoxy- - 5.73 7.47 10.10 10.87 22.34 phenol, 5-methyl-2-(1-methylethyl)- - - - - 2.08 23.28 phenol, 2, 6-dimethoxy - 15.77 17.62 19.81 7.29 23.48 t-butylhydroquinone - - - - 3.18 23.68 phenol, 2-methoxy-4-propyl- - - 1.63 2.49 3.45 25.55 benzoic acid, 4-hydroxy-3-methoxy- - 5.11 9.10 9.64 6.49 26.63 4-hydroxy-3-methoxyacetophenone - 3.50 1.94 - - 27.70 benzene, 1, 2, 3-trimrthoxy-5-methyl- - 6.94 7.14 9.14 6.38 27.84 2-propanone, 1-(4-hydroxy-3-methoxyphenyl)- - 2.83 2.98 2.87 - 28.16 4-hydroxy-benzoicaciethylester 
- 8.03 4.12 - - 29.29 benzoic acid, 4-hydroxy-3-methoxy-, ethyl ester - 8.15 3.24 2.71 - 29.31 diethyl suberate - - - - 4.58 29.71 2, 4, 6-trimethoxy benzaldehyde - - - - 2.70 30.51 4-hydroxy-3-methoxyphenylacetic acid, ethyl ester - 7.59 4.58 4.19 1.28 31.62 decanedioic acid, dimethyl ester - - - - 9.69 32.54 ethanone, 1-(4-hydroxy-3, 5-dimethoxyphenyl)- 100 10.30 4.75 3.17 - 32.90 ethyl-β-(4-hydroxy-3-methoxy-phenyl)-propionate - 2.77 5.95 4.97 3.01 33.05 3, 5-domethoxy-4-hydroxyphenylacetic acid - 1.45 4.05 4.05 - 35.59 acetic acid, diphenyl-, ethyl ester - 6.64 - 3.11 - -
[1] AZADI P, INDERWILDIA O, FARNOOD R, KING D A.Liquid fuels, hydrogen and chemicals from lignin:A critical review[J].Renewable Sustainable Energy Rev, 2013, 21:506-523. doi: 10.1016/j.rser.2012.12.022 [2] YOSHIKAWA T, YAGI T, SHINOHARA S, FUKUNAGA T, NAKASAKA Y, TAGO T, MASUDA T.Production of phenols from lignin via depolymerization and catalytic cracking[J].Fuel Process Technol, 2013, 108:69-75. doi: 10.1016/j.fuproc.2012.05.003 [3] PANDEY M P, KIM C S.Lignin depolymerization and conversion:A review of thermochemical methods[J].Chem Eng Technol, 2011, 34(1):29-41. doi: 10.1002/ceat.v34.1 [4] TOLEDANO A, SERRANO L, LABIDI J.Improving base catalyzed lignin depolymerization by avoiding lignin repolymerization[J].Fuel, 2014, 116:617-624. doi: 10.1016/j.fuel.2013.08.071 [5] YUAN Z, CHENG S, LEITCH M, XU C C.Hydrolytic degradation of alkaline lignin in hot-compressed water and ethanol[J].Bioresour Technol, 2010, 101(23):9308-9313. doi: 10.1016/j.biortech.2010.06.140 [6] MAHMOOD N, YUAN Z, SCHMIDT J, XU C C.Hydrolytic depolymerization of hydrolysis lignin:Effects of catalysts and solvents[J].Bioresour Technol, 2015, 190:416-419. doi: 10.1016/j.biortech.2015.04.074 [7] 周景辉, 谢章红, 王兴, 郑来久.木素超临界流体解聚及反应机制研究进展[J].大连工业大学学报, 2013, 32(6):426-431. http://www.cnki.com.cn/Article/CJFDTOTAL-DLQG201306010.htmZHOU Jing-hui, XIE Zhang-hong, WANG Xing, ZHENG Lai-jiu.Advance in lignin-depolymerization and reaction mechanism by supercritical fluids technique[J].J Dalian Polytech Univ, 2013, 32(6):426-431. http://www.cnki.com.cn/Article/CJFDTOTAL-DLQG201306010.htm [8] PIŃKOWSKA H, WOLAK P, ZŁOCIŃSKA A.Hydrothermal decomposition of alkali lignin in sub-and supercritical water[J].Chem Eng J, 2012, 187:410-414. doi: 10.1016/j.cej.2012.01.092 [9] KIM J Y, OH S, WANG H H, CHO T S, CHOI I G, CHOI J W.Effects of various reaction parameters on solvolytical depolymerization of lignin in sub-and supercritical ethanol[J].Chemosphere, 2013, 93(9):1755-1764. doi: 10.1016/j.chemosphere.2013.06.003 [10] TAN S S, MACFARLANE D R, UPFAL J, EDYE L A, DOHERTY W O, PATTI A F, SCOTT J L.Extraction of lignin from lignocellulose at atmospheric pressure using alkylbenzenesulfonate ionic liquid[J].Green Chem, 2009, 11(3):339-345. doi: 10.1039/b815310h [11] CHENG S, WIKS C, YUAN Z, LEITCH M, XU C C.Hydrothermal degradation of alkali lignin to bio-phenolic compounds in sub/supercritical ethanol and water-ethanol co-solvent[J].Polym Degrad Stab, 2012, 97(6):839-848. doi: 10.1016/j.polymdegradstab.2012.03.044 [12] HU J, SHEN D, WU S, ZHANG H, XIAO R.Effect of temperature on structure evolution in char from hydrothermal degradation of lignin[J].J Anal Appl Pyrolysis, 2014, 106:118-124. doi: 10.1016/j.jaap.2014.01.008 [13] SHARMA R K, WOOTEN J B, BALIGA V L, LIN X, CHAN W G, HAJALIGOL M R.Characterization of chars from pyrolysis of lignin[J].Fuel, 2004, 83(11):1469-1482. [14] LORA J H, GLASSER W G.Recent industrial applications of lignin:A sustainable alternative to nonrenewable materials[J].J Polym Environ, 2002, 10(1/2):39-48. doi: 10.1023/A:1021070006895 -





 下载:
下载: