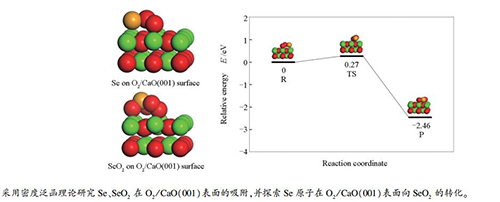
First-principles study of the adsorption and reaction of Se and SeO2 on O2/CaO(001) surface
-
摘要: 基于密度泛函理论的第一性原理和平板模型构造了最稳定的O2/CaO(001)表面,通过优化Se和SeO2在此表面可能的初始吸附结构得到最佳吸附构型,分析了Se原子在O2/CaO(001)表面向SeO2的转化。结果表明,Se原子在O2/CaO(001)表面的稳定吸附构型主要有两种,即O-Se-O和O-O-Se基团,其中,O-O-Se基团的Se终端具有一定化学活性;Se在O2/CaO(001)表面向SeO2转化所需反应能垒小于均相条件下生成SeO2所需反应能垒,表明CaO不仅作为吸附剂,也能促进Se向SeO2的转化;SeO2分子在O2/CaO(001)表面发生化学吸附时,吸附基底的部分价电子转移至SeO2分子轨道中。
-
关键词:
- 密度泛函理论 /
- 第一性原理 /
- CaO(001)表面 /
- 反应能垒
Abstract: O2/CaO(001) surface with the lowest energy was built by using the slab model and the first-principles method based on density functional theory. A series of possible adsorption configurations were optimized to get the adsorption geometries with the lowest energy for selenium (Se) and SeO2 on the O2/CaO(001) surface and the conversion of Se to SeO2 on the CaO(001) surface was then investigated. The results indicate that there are two adsorption configurations for Se atom on the O2/CaO(001) surface, viz., O-Se-O and O-O-Se groups; therein the Se terminal in O-O-Se group has a certain chemical activity. The reaction energy barrier for the heterogeneous conversion of Se and O2 to SeO2 is less than that for the homogeneous conversion, which means that CaO can not only act as an adsorbent, but also promote the conversion of Se to SeO2 as a catalyst; certain valence electrons in adsorption substrate are transferred to the orbits of SeO2 molecule when SeO2 molecule was adsorbed on the O2/CaO(001) surface.-
Key words:
- density functional theory /
- first-principles /
- CaO(001) surface /
- reaction energy barrier
-
图 6 SeO2在O2/CaO(001)表面吸附前后Se、O(2)、Casurf原子态密度图(a)吸附前O(2)、Casurf原子态密度;(b)吸附后O(2)、Casurf原子态密度;(b)吸附后Se、O(2)原子态密度
Figure 6 DOS of Se, O(2) and Casurf before/after SeO2 adsorption on the O2/CaO(001) surface: (a): DOS of O(2) and Casurf before adsorption; (b): DOS of O(2) and Casurf after adsorption; (c): DOS of Se and O(2) after adsorption
表 1 O2在CaO(001)表面O2到CaO(001)表面平均距离、O(1)-O(2)键长、吸附能及电荷分析
Table 1 Equilibrium distance, optimized O(1)-O(2) bond length, adsorption energy, Mulliken charges after the adsorption of O2 on CaO(001) surface
Surface d/nm dO(1)-O(2)/nm q(O2)/e q(Osurf)/e q(Casurf)/e Ead/eV CaO(001) - 0.123 - -5.41 5.67 - O2/CaO(001) 0.271 0.130 -0.44 -4.88 5.28 -0.96 Mulliken charges - - -0.44 0.53 -0.39 - 表 2 Se在O2/CaO(001)表面的吸附能及Se原子Mulliken布居
Table 2 Adsorption energy and Mulliken population of Se on O2/CaO(001) surface
Number Adsorption configuration Ead/eV q(Se)/e a Se on bridge -1.31 0.20 b Se on O(1)-top -1.31 0.20 c Se on O(2)-top -2.47 0.18 表 3 SeO2在O2/CaO(001)表面的吸附能及电荷分析
Table 3 Adsorption energy and Mulliken population of SeO2 on O2/CaO(001) surface
Number Adsorption configuration Ead/eV q(Se to O)/e q(SeO2)/e a O(1)-top case 1, SeO2 is parallel to O2 -1.38 0.09 -0.36 b O(1)-top case 1, SeO2 is perpendicular to O2 -2.03 0.07 -0.55 c O(1)-top case 2, O atoms of SeO2 are deviated from O(2) -2.02 0.08 -0.54 d O(1)-top case 2, O atoms of SeO2 are toward O(2) -0.48 0.05 -0.18 e bridge case 1, SeO2 is parallel to O2 -2.02 0.09 -0.51 f bridge case 1, SeO2 is perpendicular to O2 -2.03 0.07 -0.56 g bridge case 2, O atoms of SeO2 are toward O(1) -2.02 0.09 -0.53 h bridge case 2, O atoms of SeO2 are toward O(2) -1.99 0.10 -0.49 i O(2)-top case 1, SeO2 is parallel to O2 -1.35 0.07 -0.40 j O(2)-top case 1, SeO2 is perpendicular to O2 -0.49 0.06 0.17 k O(2)-top case 2, O atoms of SeO2 are toward O(1) -1.90 0.07 -0.53 l O(2)-top case 2, O atoms of SeO2 are deviated from O(1) -2.02 0.07 -0.56 a: case 1 denotes that the SeO2 on O2/CaO (001) surface perpendicula;
b: case 2 denotes that the SeO2 on O2/CaO (001) surface parallel -
[1] TANG Q, LIU G J, ZHOU C C, SUN R Y. Distribution of trace elements in feed coal and combustion residues from two coal-fired power plants at Huainan, Anhui, China[J]. Fuel, 2013, 107(9):315-322. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=66169c6bbaf0f92c141e88cab7a9d332 [2] 刘迎晖, 郑楚光, 游小清, 郭欣.燃煤过程中易挥发有毒痕量元素的相互作用[J].燃烧科学与技术, 2001, 7(4):243-247. doi: 10.3321/j.issn:1006-8740.2001.04.007LIU Ying-hui, ZHENG Chu-guang, YOU Xiao-qing, GUO Xin. Interaction between most volatile toxic trace elements during coal combustion[J]. J Combust Sci Technol, 2001, 7(4):243-247. doi: 10.3321/j.issn:1006-8740.2001.04.007 [3] 王臣.煤燃烧中硒与锡的反应机理量子化学研究[D].武汉: 华中科技大学, 2005. http://cdmd.cnki.com.cn/Article/CDMD-10487-2006038926.htmWANG Chen. Quantum chemistry study on the reaction mechanism of trace element Se and Sn in the coal combustion process[D]. Wuhan: Huazhong University of Science & Technology, 2005. http://cdmd.cnki.com.cn/Article/CDMD-10487-2006038926.htm [4] ANDREN A W, KLEIN D H, TALMI Y. Selenium in coal-fired steam plant emissions[J]. Environ Sci Technol, 1975, 9(9):856-858. doi: 10.1021/es60107a002 [5] 秦海波, 朱建明, 朱咏喧, 雷磊.大气环境中硒的存在形式、来源及通量[J].地球与环境, 2009, 37(3):304-314. http://d.old.wanfangdata.com.cn/Periodical/dzdqhx200903018QIN Hai-bo, ZHU Jian-ming, ZHU Yong-xuan, LEI Lei. Advances in research on atmospheric selenium[J]. Earth Environ, 2009, 37(3):304-314. http://d.old.wanfangdata.com.cn/Periodical/dzdqhx200903018 [6] 李玉忠.中温脱硫过程联合脱除痕量硒、砷的实验研究[D].北京: 清华大学, 2006. http://cdmd.cnki.com.cn/Article/CDMD-10003-2007190725.htmLI Yu-zhong. Experimental study on simultaneous removal of trace selenium and arsenic in flue gas desulphurization within medium temperature range[D]. Beijing: Tsinghua University, 2006. http://cdmd.cnki.com.cn/Article/CDMD-10003-2007190725.htm [7] 张月, 王春波, 刘慧敏, 孙喆, 李文瀚, 张永生, 潘伟平.金属氧化物吸附剂干法脱除气相As2O3实验研究[J].燃料化学学报, 2015, 43(4):476-482. doi: 10.3969/j.issn.0253-2409.2015.04.016ZHANG Yue, WANG Chun-bo, LIU Hui-min, SUN Zhe, LI Wen-han, ZHANG Yong-sheng, PAN Wei-ping. Removal of gas-phase As2O3 in dry process by metal oxide adsorbents[J]. J Fuel Chem Technol, 2015, 43(4):476-482. doi: 10.3969/j.issn.0253-2409.2015.04.016 [8] 黄亚继, 金保升, 仲兆平, 肖睿, 周宏仓.固体添加剂对煤气化过程中痕量元素的控制研究[J].环境科学学报, 2005, 25(4):507-511. doi: 10.3321/j.issn:0253-2468.2005.04.014HUANG Ya-ji, JIN Bao-sheng, ZHONG Zhao-ping, XIAO rui, ZHOU Hong-cang. Effects of solid additives on the control of trace elements during coal gasification[J]. Acta Sci Circum, 2005, 25(4):507-511. doi: 10.3321/j.issn:0253-2468.2005.04.014 [9] 程俊峰, 韩军, 刘迎辉, 曾汉才, 徐明厚, 雒昆利.分级燃烧中固体吸附剂对痕量金属排放的影响[J].环境科学, 2001, 22(6):34-38. http://d.old.wanfangdata.com.cn/Periodical/hjkx200106007CHENG Jun-feng, HAN Jun, LIU Ying-hui, CENG Han-cai, XU Ming-hou, LUO Kun-li. Effects of air staging with absorbents on trace metal during coal combustion[J]. Environ Sci, 2001, 22(6):34-38. http://d.old.wanfangdata.com.cn/Periodical/hjkx200106007 [10] 张军营, 任德贻, 钟秦, 徐复铭, 张衍国, 尹金双.固硫剂对煤燃烧过程中硒挥发性的抑制作用[J].环境科学, 2001, 22(3):100-103. doi: 10.3321/j.issn:0250-3301.2001.03.022ZHANG Jun-ying, REN De-yi, ZHONG Qin, XU Fu-ming, ZHANG Yan-guo, YIN Jin-shuang. Retention of selenium volatility using lime in coal combustion[J]. Environ Sci, 2001, 22(3):100-103. doi: 10.3321/j.issn:0250-3301.2001.03.022 [11] 熊全军, 邱建荣, 徐朝芬, 王泉海, 刘豪, 陈永利, 徐志英.氧燃烧方式下重金属Se挥发行为的研究[J].工程热物理学报, 2006, 27(2):195-198. http://d.old.wanfangdata.com.cn/Periodical/gcrwlxb2006z2051XIONG Quan-jun, QIU Jian-rong, XU Chao-fen, WANG Quan-hai, LIU Hao, CHEN Yong-li, XU Zhi-ying. Study on the volatilization behavior of Se under oxygen-combustion atmosphere[J]. J Eng Therm, 2006, 27(2):195-198. http://d.old.wanfangdata.com.cn/Periodical/gcrwlxb2006z2051 [12] GALBREATH K C, ZYGARLICKE C J. Mercury transformations in coal combustion flue gas[J]. Fuel Process Technol, 2000, 65(99):289-310. https://www.sciencedirect.com/science/article/pii/S0378382099001022 [13] WANG L Z. Adsorption of O2 on the CaO and SrO(100) surfaces:A first-principles study[J]. Appl Surf Sci, 2011, 257(13):5499-5502. doi: 10.1016/j.apsusc.2010.12.126 [14] FAN Y M, ZHUO Y Q, LOU Y, ZHU Z W, LI L L. SeO2 adsorption on CaO surface:DFT study on the adsorption of a single SeO2 molecule[J]. Appl Surf Sci, 2017, 413:366-371. doi: 10.1016/j.apsusc.2017.03.196 [15] FAN Y M, ZHUO Y Q, LI L L. SeO2 adsorption on CaO surface:DFT and experimental study on the adsorption of multiple SeO2 molecules[J]. Appl Surf Sci, 2017, 420:465-471. doi: 10.1016/j.apsusc.2017.04.233 [16] DELLEY B. An all-electron numerical method for solving the local density functional for polyatomic molecules[J]. J Chem Phys, 1990, 92(1):508-511. doi: 10.1063/1.458452 [17] DELLEY B. From molecules to solids with the DMol3 approach[J]. J Chem Phys, 2000, 113(18):7756-7764. doi: 10.1063/1.1316015 [18] PERDEW J P, CHEVARY J A, VOSKO S H, JACKSON K A, PERDERSON M R, SINGQH D J, FIOLHAIS C. Atoms, molecules, solids, and surfaces:Applications of the generalized gradient approximation for exchange and correlation[J]. Phys Rev B Condens Matter, 1993, 46(11):6671-6687. https://www.ncbi.nlm.nih.gov/pubmed/10002368 [19] VANDERBILT D. Soft self-consistent pseudopotentials in a generalized eigenvalue formalism[J]. Phys Rev B, 1990, 41(11):7892-7895. doi: 10.1103/PhysRevB.41.7892 [20] BLOWERS P, BO G K. The adsorption of mercury-species on relaxed and rumpled CaO(001) surfaces investigated by density functional theory[J]. J Mol Model, 2011, 17(3):505-514. doi: 10.1007/s00894-010-0741-5 [21] GOVIND N, PETERSEN M, FITZGERALD G, KING-SMITH D, ANDZELM J. A generalized synchronous transit method for transition state location[J]. Comp Mater Sci, 2003, 28(2):250-258. doi: 10.1016/S0927-0256(03)00111-3 [22] SHAH M H, IQBAL J, SHAHEEN N, KHAN N, CHOUDHARY M A, AKHTER G. Assessment of background levels of trace metals in water and soil from a remote region of Himalaya[J]. Environ Monit Assess, 2012, 184(3):1243-1252. doi: 10.1007/s10661-011-2036-4 [23] DENISALPIZAR O, STOECKLIN T, HALVICK P, DUBERNET M L, MARINAKIS S. Potential energy surface and rovibrational energy levels of the H2-CS van der Waals complex[J]. J Chem Phys, 2012, 137(23):234-301. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=9b2eb6ccd250c1aa2f021033fa93a190 [24] XIN G, ZHAO P F, ZHENG C G. Theoretical study of different speciation of mercury adsorption on CaO(001) surface[J]. P Combust Inst, 2009, 32(2):2693-2699. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=e5f06f5b58cc86db05a8462fc737ac88 [25] 吴令男.燃料在循环流化床锅炉燃烧过程中挥发分氮的迁移规律研究[D].北京: 华北电力大学, 2017.WU Ling-nan. Study on the conversion mechanism of volatile nitrogen during fuel combustion in circulating fluidized bed boilers[D]. Beijing: North China Electric Power University, 2017. [26] 陈玉红, 刘婷婷, 张梅玲, 元丽华, 张材荣. H2分子在Mg3N2表面吸附的第一性原理研究[J].化学学报, 2017, 75(7):708-714. http://d.old.wanfangdata.com.cn/Periodical/hxxb201707008CHEN Yu-hong, LIU Ting-ting, ZHANG Mei-ling, YUAN Li-hua, ZHANG Cai-rong. First principles study on the adsorption of H2 molecules on Mg3N2 surface[J]. Acta Chim Sinica, 2017, 75(7):708-714. http://d.old.wanfangdata.com.cn/Periodical/hxxb201707008 -





 下载:
下载: