-
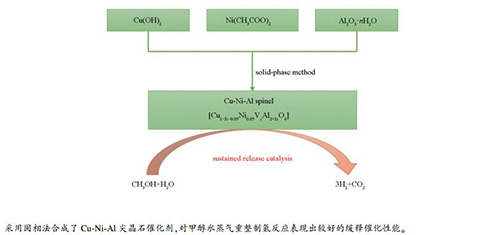
摘要: 以氢氧化铜、醋酸镍和拟薄水铝石为原料,通过固相法合成了Cu-Ni-Al尖晶石催化剂。采用N2物理吸附、XRD、H2-TPR和XPS等表征方法,研究Cu/Ni/Al的物质的量比和焙烧温度对催化剂的比表面积、物相、还原性能以及表面性质的影响,并以甲醇水蒸气重整制氢为探针反应,考察催化剂的缓释催化性能。结果表明,随着焙烧温度的升高,Cu-Ni-Al催化剂的尖晶石含量增加,但尖晶石晶粒增大,且比表面积下降。不同的焙烧温度和Cu/Ni/Al物质的量比,所得催化剂的比表面积、还原性能和表面性质不同,从而表现出不同的缓释催化性能。与计量比Cu/Al=1:2的合成比较,Cu/Al=1:3形成了非计量比的富Al尖晶石固溶体,生成的晶体粒子小、比表面积和孔容大、难还原的尖晶石部分增多,呈现出更好的缓释催化性能。甲醇制氢反应性能评价结果显示,Cu-Ni-Al尖晶石在反应条件下逐渐释放活性铜而催化反应的进行,其中,CNA3-1000催化剂表现中最高的催化活性和稳定性。
-
关键词:
- Cu-Ni-Al尖晶石 /
- 固相法 /
- 甲醇重整 /
- 氢气
Abstract: Using copper hydroxide, nickel acetate and pseudoboehmite as materials, the Cu-Ni-Al spinel catalysts were synthesized by the solid-phase method. The effects of Cu/Ni/Al molar ratio and calcination temperature on specific surface area, phase composition, reduction performance and surface property of Cu-Ni-Al spinel catalysts were characterized by BET, XRD, H2-TPR and XPS. Moreover, the sustained release catalytic performances of Cu-Ni-Al spinel samples for methanol steam reforming were tested. The obtained results indicated that with increasing the calcination temperature, the content of Cu-Ni-Al spinel increased, but the size of spinel particles increased and the specific surface area decreased. Change of the calcination temperature and Cu/Ni/Al molar ratio led to different specific surface area, reduction performance and surface property of Cu-Ni-Al spinel catalysts, thus showing different sustained release catalytic performance. Comparing with those of stoichiometric ratio of Cu/Al=1:2, spinel solid solutions with smaller particle size, higher specific surface area and pore volume, more hardly-reducible spinel and better sustained release catalytic performance were obtained with the nonstoichiometric ratio of Cu/Al=1:3. The results of catalyst evaluation indicated that active copper species were released from Cu-Ni-Al spinel lattice and thus took part in the catalytic action. Among the prepared catalysts, CNA3-1000 catalyst showed the highest catalytic activity and stability.-
Key words:
- Cu-Ni-Al spinel /
- solid-phase method /
- methanol steam reforming /
- hydrogen
1) 本文的英文电子版由Elsevier出版社在ScienceDirect上出版(http://www.sciencedirect.com/science/journal/18725813). -
表 1 CNA2-T和CNA3-T催化剂的物理化学性质
Table 1 Physicochemical properties of CNA2-T and CNA3-T catalysts
CNA2-900 CNA2-1000 CNA2-1100 CNA3-900 CNA3-1000 CNA3-1100 A/(m2· g-1) 37.4 19.1 17.0 51.0 30.5 18.2 v/(cm3 · g-1) 0.302 0.176 0.082 0.274 0.223 0.106 Xnon-spinel/% a 35.2 13.4 8.9 35.1 13.8 6.3 Xspinel/% b 64.8 86.6 91.1 64.9 86.2 93.7 XH/% c 25.2 16.2 15.9 42.1 39.9 19.6 xd 0.092 0.033 0.022 0.158 0.114 0.099 dspinel/nme 10.8 25.3 32.8 8.5 15.7 30.8 a/ nmf 0.8064 0.8079 0.8080 0.8046 0.8054 0.8078 dCu/nmg 15.7 15.1 16.2 14.4 10.9 15.2 a: the molar ratio of Cu in non-spinel phase to total Cu as derived from H2-TPR (Figure 3); b: the molar ratio of Cu in spinel phase to total Cu as derived from H2-TPR (Figure 3); c: the molar ratio of hardly-reducible spinel; d: x in Cu1-3x-0.05Ni0.05VxAl2+2xO4; e: the crystallite size of spinels, calculated using the Scherrer equation from the XRD patterns (Figure 2); f: cell parameter of spinel; g: the crystallite size of Cu in tested samples calculated using the Scherrer equation from the XRD patterns (Figure 9) -
[1] WANG M Y, WANG Z, GONG X Z, GUO Z C. The intensification technologies to water electrolysis for hydrogen production-A review[J]. Renewable Sustainable Energy Rev, 2014, 29:573-588. doi: 10.1016/j.rser.2013.08.090 [2] BELL T E, TORRENTE-MURCIANO L. H2 Production via ammonia decomposition using non-noble metal catalysts:A review[J]. Top Catal, 2016, 59(15):1438-1457. http://cn.bing.com/academic/profile?id=d078c0d2ae64e434df25f504bc980900&encoded=0&v=paper_preview&mkt=zh-cn [3] PAL D B, CHAND R, UPADHYAY S N, MISHRA P K. Performance of water gas shift reaction catalysts:A review[J]. Renewable Sustainable Energy Rev, 2018, 93:549-565. doi: 10.1016/j.rser.2018.05.003 [4] SÁ S, SILVA H, BRANDÃO L, SOUSA J M, MENDES A. Catalysts for methanol steam reforming:A review[J]. Appl Catal B:Environ, 2010, 99(1):43-57. https://www.sciencedirect.com/science/article/pii/S0926337310002584 [5] LIN L L, ZHOU W, GAO R, YAO S Y, ZHANG X, XU W Q, ZHENG S J, JIANG Z, YU Q L, LI Y W, SHI C, WEN X D, MA D. Low-temperature hydrogen production from water and methanol using Pt/α-MoC catalysts[J]. Nature, 2017, 544:80-97. doi: 10.1038/nature21672 [6] BAGHERZADEH S B, HAGHIGHI M. Plasma-enhanced comparative hydrothermal and coprecipitation preparation of CuO/ZnO/Al2O3 nanocatalyst used in hydrogen production via methanol steam reforming[J]. Energy Convers Manage, 2017, 142:452-465. doi: 10.1016/j.enconman.2017.03.069 [7] SANCHES S G, HUERTAS FLORES J, PAIS DA SILVA M I. Influence of aging time on the microstructural characteristics of a Cu/ZnO-based catalyst prepared by homogeneous precipitation for use in methanol steam reforming[J]. React Kinet Mech Catal, 2017, 121(2):473-485. doi: 10.1007/s11144-017-1161-7 [8] 杨淑倩, 张娜, 贺建平, 张磊, 王宏浩, 白金, 张健, 刘道胜, 杨占旭. Ce的浸渍顺序对Cu/Zn-Al水滑石衍生催化剂用于甲醇水蒸气重整制氢性能的影响[J].燃料化学学报, 2018, 46(4):479-488. doi: 10.3969/j.issn.0253-2409.2018.04.014YANG Shu-qian, ZHANG Na, HE Jian-ping, ZHANG Lei, WANG Hong-hao, BAI Jin, ZHANG Jian, LIU Dao-sheng, YANG Zhan-xu. Effect of impregnation sequence of Ce on the performance of Cu/Zn-Al catalysts derived from hydrotalcite precursor in methanol steam reforming[J]. J Fuel Chem Technol, 2018, 46(4):479-488. doi: 10.3969/j.issn.0253-2409.2018.04.014 [9] 杨淑倩, 贺建平, 张娜, 隋晓伟, 张磊, 杨占旭.稀土掺杂改性对Cu/ZnAl水滑石衍生催化剂甲醇水蒸气重整制氢性能的影响[J].燃料化学学报, 2018, 46(2):179-188. doi: 10.3969/j.issn.0253-2409.2018.02.007YANG Shu-qian, HE Jian-ping, ZHANG Na, SUI Xiao-wei, ZHANG Lei, YANG Zhan-xu. Effect of rare-earth element modification on the performance of Cu/ZnAl catalysts derived from hydrotalcite precursor in methanol steam reforming[J]. J Fuel Chem Technol, 2018, 46(2):179-188. doi: 10.3969/j.issn.0253-2409.2018.02.007 [10] TAHAY P, KHANI Y, JABARI M, BAHADORAN F, SAFARI N. Highly porous monolith/TiO2 supported Cu, Cu-Ni, Ru, and Pt catalysts in methanol steam reforming process for H2 generation[J]. Appl Catal A:Gen, 2018, 554:44-53. doi: 10.1016/j.apcata.2018.01.022 [11] XI H J, HOU X N, LIU Y J, QING S J, GAO Z X. Cu-Al spinel oxide as an efficient catalyst for methanol steam reforming[J]. Angew Chem Int Ed, 2014, 53(44):11886-11889. doi: 10.1002/anie.201405213 [12] LIU Y J, QING S J, HOU X N, QIN F J, WANG X, GAO Z X, XIANG H W. Temperature dependence of Cu-Al spinel formation and its catalytic performance in methanol steam reforming[J]. Catal Sci Technol, 2017, 7(21):5069-5078. doi: 10.1039/C7CY01236E [13] 覃发玠, 刘雅杰, 庆绍军, 侯晓宁, 高志贤.甲醇制氢铜铝尖晶石缓释催化剂的研究-不同铜源合成的影响[J].燃料化学学报, 2017, 45(12):1481-1488. doi: 10.3969/j.issn.0253-2409.2017.12.010QIN Fa-jie, LIU Ya-jie, QING Shao-jun, HOU Xiao-ning, GAO Zhi-xian. Cu-Al spinel as a sustained release catalyst for H2 production from methanol steam reforming:Effects of different copper sources[J]. J Fuel Chem Technol, 2017, 45(12):1481-1488. doi: 10.3969/j.issn.0253-2409.2017.12.010 [14] ZHOU R S, SNYDER R L. Structures and transformation mechanisms of theη, γ and θ transition aluminas[J]. Acta Cryst, 1991, 47(5):617-630. doi: 10.1107/S0108768191002719 [15] AREÁN C O, VIÑUELA J S D. Structural study of copper-nickel aluminate (CuxNi1-xAl2O4) spinels[J]. J Solid State Chem, 1985, 60(1):1-5. doi: 10.1016-0022-4596(85)90156-2/ [16] STROHMEIER B R, LEYDEN D E, FIELD R S, HERCULES D M. Surface spectroscopic characterization of Cu/Al2O3 catalysts[J]. J Catal, 1985, 94(2):514-530. doi: 10.1016/0021-9517(85)90216-7 [17] FIGUEIREDO R T, MARTÍNEZ-ARIAS A, GRANADOS M L, FIERRO J L G. Spectroscopic evidence of Cu-Al interactions in Cu-Zn-Al mixed oxide catalysts used in CO hydrogenation[J]. J Catal, 1998, 178(1):146-152. doi: 10.1006/jcat.1998.2106 [18] WAGNER C D, DAVIS L E, ZELLER M V, TAYLOR J A, RAYMOND R H, GALE L H. Empirical atomic sensitivity factors for quantitative analysis by electron spectroscopy for chemical analysis[J]. Surf Interface Anal, 1981, 3(5):211-225. doi: 10.1002/(ISSN)1096-9918 [19] NG K T, HERCULES D M. Studies of nickel-tungsten-alumina catalysts by X-ray photoelectron spectroscopy[J]. J Phys Chem, 1976, 80(19):2094-2102. doi: 10.1021/j100560a009 -





 下载:
下载: