Preparation of Zr-based perovskite supported Fe2O3 catalyst and its performance in the reverse water gas shift reaction
-
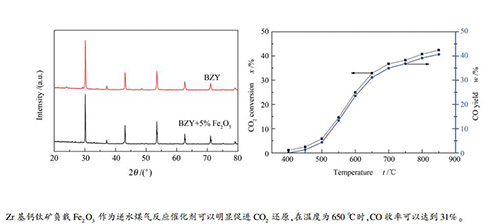
摘要: 采用固相反应法制备了钙钛矿结构的BaZr0.9Y0.1O3,并用BaZr0.9Y0.1O3作为载体负载Fe2O3,通过X射线衍射分析(XRD)、扫描电子显微镜(SEM)观察负载型催化剂的晶相结构和微观形貌,同时考察了制备的催化剂的逆水煤气反应催化活性。结果表明,BaZr0.9Y0.1O3粉体1200℃煅烧5h时,负载型催化剂具有较好的催化活性;BaZr0.9Y0.1O3对逆水煤气反应有一定的催化作用,负载少量的Fe2O3催化剂可以明显促进CO2还原,在空速为1.13h-1,温度为650℃时,CO收率可以达到31%;催化剂经过长时间运行催化效果良好,制备的催化剂活性较稳定。Abstract: BaZr0.9Y0.1O3 with perovskite structure was prepared by solid-phase reaction method and used as support to prepare Fe2O3 based catalysts. X-ray diffraction analysis (XRD) and scanning electron microscopy (SEM) were used to observe the crystal phase structure and microscopic morphology of the prepared catalysts. The catalyst performance for the reverse water gas shift reaction was also investigated. The results showed that the supported catalyst has better catalytic activity when the BaZr0.9Y0.1O3 powder was calcined at 1200℃ for 5 h. BaZr0.9Y0.1O3 has an obvious catalytic effect on the reverse water gas reaction, and the Fe2O3-supported catalyst can significantly promote CO2 reduction. Moreover, loading small amount of Fe2O3 has apparent effect on the reactivity of the catalyst. When the space velocity was 1.13 h-1, the CO yield can reach 31% at 650℃. Carbon deposition on the catalyst during the CO2 reduction process was taking place in a low rate, leading to a significant increase in the CO yield in the process of cooling-down experiment. In addition, the activity of the catalyst did not significantly decrease after a long period of reaction, which proved that the activity of the prepared catalyst was relatively stable.
-
Key words:
- Fe2O3 /
- catalyst /
- Zr-based catalyst /
- reverse water gas reaction
-
表 1 CO收率随负载量的变化
Table 1 CO yield of different Fe2O3 loading catalysts at 650 ℃
Catalyst BZY BZY+3%Fe2O3 BZY+5% Fe2O3 BZY+8% Fe2O3 BZY+15% Fe2O3 BZY+20% Fe2O3 CO yield w/% 12.88 32.24 31.14 31.55 26.03 23.88 -
[1] 程薇.美国化学协会全国研讨会主题是CO2转化制燃料的技术[J].石油炼制与化工, 2014, 45(1):76. http://www.cnki.com.cn/Article/CJFDTOTAL-SYLH201401029.htmCHENG Wei. The theme of the national symposium of the American chemical society is the technology of CO2 conversion to fuel[J]. Pet Process Petrochem, 2014, 45(1):76. http://www.cnki.com.cn/Article/CJFDTOTAL-SYLH201401029.htm [2] DAZA Y A, KENT R A, YUNG M M, KUHN J N. Carbon dioxide conversion by reverse water-gas shift chemical looping on perovskite-type oxides[J]. Ind Eng Chem Res, 2014, 53(14):5828-5837. doi: 10.1021/ie5002185 [3] OSHIMA K, SHINAGAWA T, NOGAMI Y, MANBE R, OGO S, SEKINE Y. Low temperature catalytic reverse water gas shift reaction assisted by an electric field[J]. Catal Today, 2014, 232:27-32. doi: 10.1016/j.cattod.2013.11.035 [4] PETTIGREW D J, TRIMM D L. The effects of rare earth oxides on the reverse water-gas shift reaction on palladium/alumina[J]. Cataly Lett, 1994, 28(2):313-319. doi: 10.1007/BF00806061 [5] GOGATE M R, DAVIS R J. Comparative study of CO and CO2, hydrogenation over supported Rh-Fe catalysts[J]. Catal Commun, 2010, 11(10):901-906. doi: 10.1016/j.catcom.2010.03.020 [6] KIM S S, PARK K H, HONG S C. A study of the selectivity of the reverse water-gas-shift reaction over Pt/TiO2 catalysts[J]. Fuel Process Technol, 2013, 108:47-54. doi: 10.1016/j.fuproc.2012.04.003 [7] WANG X, SHI H, KWAK J H, SZANYI J. Mechanism of CO2 hydrogenation on Pd/Al2O3 catalysts:Kinetics and transient DRIFTS-MS studies[J]. ACS Catal, 2015, 5(11):6337-6349. doi: 10.1021/acscatal.5b01464 [8] LIU Y, LIU D. Study of bimetallic Cu-Ni/γ-Al2O3 catalysts for carbon dioxide hydrogenation[J]. Int J Hydrogen Energy, 1999, 24(4):351-354. doi: 10.1016/S0360-3199(98)00038-X [9] RONDA-LLORET M, RICO-FRANCES S, SEPULVEDA-ESCRIBANO A, RAMOS-FERNANDEZ E V. CuO/CeO2 catalyst derived from metal organic framework for reverse water-gas shift reaction[J]. Appl Catal A:Gen, 2018, 562:28-36. doi: 10.1016/j.apcata.2018.05.024 [10] 何孝祥, 顾雄毅, 范琛, 朱贻安. Fe3O4表面逆水煤气反应的DFT研究[J].华东理工大学学报(自然科学版), 2011, 37(4):424-429. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK201101575707HE Xiao-xiang, GU Xiong-yi, FAN Chen, ZHU Yi-an. DFT study of reverse water-gas shift reaction on Fe3O4 surface[J]. J East China Univ Sci Technol, 2011, 37(4):424-429. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=QK201101575707 [11] 代必灿, 周桂林.逆水煤气变换(RWGS)催化剂研究进展[J].化工进展, 2017, 36(7):2473-2480. http://d.old.wanfangdata.com.cn/Periodical/hgjz201707018DAI Bi-can, ZHOU Gui-lin. Perspective on catalyst investigation for reverse water-gas shift reaction (RWGS)[J]. Chem Ind Eng Process, 2017, 36(7):2473-2480. http://d.old.wanfangdata.com.cn/Periodical/hgjz201707018 [12] 李军, 崔凤霞, 李荣.二氧化碳还原技术研究进展[J].精细石油化工, 2017, 34(2):75-82. doi: 10.3969/j.issn.1003-9384.2017.02.018LI Jun, CUI Feng-xia, LI Rong. Research progress on carbon dioxide reduction technology[J]. Spec Petrochem, 2017, 34(2):75-82. doi: 10.3969/j.issn.1003-9384.2017.02.018 [13] KIM D H, HAN S W, YOON H S, KIM Y D. Reverse water gas shift reaction catalyzed by Fe nanoparticles with high catalytic activity and stability[J]. J Ind Eng Chem, 2015, 23:67-71. doi: 10.1016/j.jiec.2014.07.043 [14] ERTL G, KNOTZINGER H, SCHUTH F, WEITCAMP J. Handbook of Heterogeneous Catalysis[M]. Germany:VCH Publishers, 2007. [15] KIM D H, PARK J L, PARK E J, KIM Y D, Uhm S. Dopant effect of barium zirconate-based perovskite-type catalysts for the intermediate-temperature reverse water gas shift reaction[J]. ACS Catal, 2014, 4(9):3117-3122. doi: 10.1021/cs500476e [16] 王同同.全陶瓷双相中空纤维膜的制备及在NH3分解制氢中的应用[D].山东: 山东理工大学, 2017.WANG Tong-Tong. Preparation of dual phase ceramic hollow fiber membranes and application in NH3 decomposition for hydrogen production[D]. Shandong: Shandong University of Technology. [17] 曹加锋, 朱志文, 刘卫.钙钛矿结构质子导体基固体氧化物燃料电池电解质研究进展[J].硅酸盐学报, 2015, 43(6):734-740. http://d.old.wanfangdata.com.cn/Periodical/gsyxb201506005CAO Jia-feng, ZHU Zhi-wen, LIU Wei. Review on perovskite electrolyte for proton-conducting solid oxide fuel cells[J]. J Chin Silic Soc, 2015, 43(6):734-740. http://d.old.wanfangdata.com.cn/Periodical/gsyxb201506005 -





 下载:
下载: