Pt/HZSM-5 catalyst synthesized by atomic layer deposition for aqueous-phase hydrogenation of levulinic acid to valeric acid
-
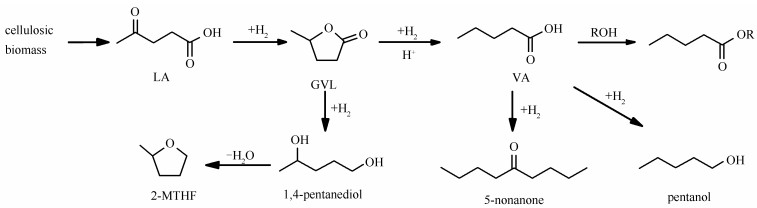
摘要: 利用原子层沉积(ALD)技术制备出Pt/HZSM-5催化剂,并用于乙酰丙酸(LA)水相加氢制戊酸(VA)。在HZSM-5上沉积五个循环时的5Pt/HZSM-5催化剂,其VA收率高达91.4%,且具有较高的稳定性。研究表明,Pt加氢位点和HZSM-5酸性位点距离越近越有利于VA的选择性生成。通过延长沉积的扩散时间,ALD可将Pt沉积到HZSM-5的微孔通道中,但对HZSM-5的微孔结构和酸性位点影响较小,这体现出ALD在保护HZSM-5结构上的优势。随着ALD沉积Pt循环数的增加,Pt纳米颗粒的平均粒径、表面Pt的电子状态、HZSM-5表面酸位点都没有发生明显的变化,分子筛孔道中的Pt比例则逐渐降低,这导致VA生成的TOF降低。同时,也通过浸渍法制备了负载在HZSM-5上的Pt催化剂作为对比,结果表明,浸渍法导致HZSM-5的孔结构受损,形成了更多的微孔,表面酸性位点数目降低,其催化活性、VA选择性和稳定性都显著低于ALD制备的催化剂。Abstract: A Pt/HZSM-5 catalyst was prepared by atomic layer deposition (ALD) for aqueous-phase hydrogenation of levulinic acid (LA) to valeric acid (VA). 5Pt/HZSM-5 produced with 5 cycles of Pt ALD was identified as a highly active and stable bifunctional catalyst, and a high yield of VA (91.4%) was achieved in aqueous solution. A close interaction between Pt and acid sites of HZSM-5 is favor for the selective generation of VA. The microporous structure and the acid sites of HZSM-5 were not changed after Pt ALD, and some Pt nanoparticles were located in the micropore channel of HZSM-5. This reveals that the Pt ALD has the advantage to protect the structure of zeolite. The average particle size of Pt nanoparticles, electric state of surface Pt, and surface acid sites are nearly not changed with the increase of Pt ALD cycle number. However, the ratio of Pt in the pore channel to that out of the pore decreases with the increase of ALD cycle numbers, resulting in a decrease of TOF of VA yield. For comparison, Pt nanoparticles supported on HZSM-5 were also produced by impregnation. But the pore structure of HZSM-5 was damaged, and more micropore were formed by impregnation method for Pt loading. Moreover, it exhibited very low catalytic activity, selectivity of VA, and stability.
-
Key words:
- Pt /
- atomic layer deposition /
- levulinic acid /
- hydrogentation /
- valeric acid
-
Table 1 Catalytic performance of different catalysts in aqueous LA hydrogenationa

Table 2 Physicochemical characteristics of HZSM-5 and HZSM-5 supported Pt catalystsa

Table 3 Summary of XPS results for Pt 4f of the xPt/HZSM-5 catalysts

-
[1] RAGAUSKAS A J, WILLIAMS C K, DAVISON B H, BRITOVSEK G, CAIRNEY J, ECKERT C A, FREDERICK W J, HALLETT J P, LEAK D J, LIOTTA C L, MIELENZ J R, MURPHY R, TEMPLER R, TSCHAPLINSK T. The path forward for biofuels and biomaterials[J]. Science, 2006, 311 (5760): 484-489. doi: 10.1126/science.1114736 [2] YUAN J, LI S S, YU L, LIU Y M, CAO Y, HE H Y, FAN K N. Copper-based catalysts for the efficient conversion of carbohydrate biomass into γ-valerolactone in the absence of externally added hydrogen[J]. Energy Environ Sci, 2013, 6 (11): 3308-3313. doi: 10.1039/c3ee40857d [3] LUO W H, DEKA U, BEALE A M, VAN ECK E R H, BRUIJNINCX P C A, WECKHUYSEN B M. Ruthenium-catalyzed hydrogenation of levulinic acid: Influence of the support and solvent on catalyst selectivity and stability[J]. J Catal, 2013, 301 : 175-186. doi: 10.1016/j.jcat.2013.02.003 [4] LANGE J P, PRICE R, AYOUB P M, LOUIS J, PETRUS L, CLARKE L, GOSSELINK H. Valeric biofuels: A platform of cellulosic transportation fuels[J]. Angew Chem Int Ed, 2010, 49 (26): 4479-4483. doi: 10.1002/anie.201000655 [5] GALLETTI A M R, ANTONETTI C, DE LUISE V, MARTINELLI M. A sustainable process for the production of γ-valerolactone by hydrogenation of biomass-derived levulinic acid[J]. Green Chem, 2012, 14 (3): 688-694. doi: 10.1039/c2gc15872h [6] PAN T, DENG J, XU Q, XU Y, GUO Q X, FU Y. Catalytic conversion of biomass-derived levulinic acid to valerate esters as oxygenated fuels using supported ruthenium catalysts[J]. Green Chem, 2013, 15 (10): 2967-2974. doi: 10.1039/c3gc40927a [7] PACE V, HOYOS P, CASTOLDI L, DOMINGUEZ D E MARIA P, ALCANTARA A R. 2-methyltetrahydrofuran (2-MeTHF): A biomass-derived solvent with broad application in organic chemistry[J]. ChemSusChem, 2012, 5 (8): 1369-1379. doi: 10.1002/cssc.v5.8 [8] SERRANO-RUIZ J C, WANG D, DUMESIC J A. Catalytic upgrading of levulinic acid to 5-nonanone[J]. Green Chem, 2010, 12 (4): 574-577. doi: 10.1039/b923907c [9] YAN K, LAFLEUR T, WU X, CHAI J J, WU G S, XIE X M. Cascade upgrading of γ-valerolactone to biofuels[J]. Chem Commun, 2015, 51 (32): 6984-6987. doi: 10.1039/C5CC01463H [10] LUO W H, BRUIJNINCX, P C A, WECKHUYSEN B M. Selective, one-pot catalytic conversion of levulinic acid to pentanoic acid over Ru/HZSM-5[J]. J Catal, 2014, 320 : 33-41. doi: 10.1016/j.jcat.2014.09.014 [11] KON K, ONODERA W, SHIMIZU K I. Selective hydrogenation of levulinic acid to valeric acid and valeric biofuels by a Pt/HMFI catalyst[J]. Catal Sci Technol, 2014, 4 (9): 3227-3234. doi: 10.1039/C4CY00504J [12] SUN P, GAO G, ZHAO Z L, XIA C G, LI F W. Stabilization of cobalt catalysts by embedment for efficient production of valeric biofuel[J]. ACS Catal, 2014, 4 (11): 4136-4142. doi: 10.1021/cs501409s [13] HUBER G W, IBORRA S, CORMA A. Synthesis of transportation fuels from biomass: Chemistry, catalysts, and engineering[J]. Chem Rev, 2006, 106 (9): 4044-4098. doi: 10.1021/cr068360d [14] ZHANG B, ZHU Y L, DING G Q, ZHENG H Y, LI Y W. Selective conversion of furfuryl alcohol to 1, 2-pentanediol over a Ru/MnOxcatalyst in aqueous phase[J]. Green Chem, 2012, 14 (12): 3402-3409. doi: 10.1039/c2gc36270h [15] DENDOOVEN J, GORIS B, DEVLOO-CASIER K, LEVRAU E, BIERMANS E, BAKLANOV M R, LUDWIG K F, VOORT P V D, BALS S, DETAVERNIER C.Tuning the pore size of ink-bottle mesopores by atomic layer deposition[J]. Chem Mater, 2012, 24 (11): 1992-1994. doi: 10.1021/cm203754a [16] LEUS K, DENDOOVEN J, TAHIR N, RAMACHANDRAN R, MELEDINA M, TURNER S, VAN TENDELOO G, GOEMAN J, VAN DER EYCKEN J, DETAVERNIER C, VAN DER VOORT P. Atomic layer deposition of Pt nanoparticles with in the cages of MIL-101: A mild and recyclable hydrogenation catalyst[J]. Nanomaterials, 2016, 6 (3): 45. doi: 10.3390/nano6030045 [17] GEORGE S M, STEVEN M G. Atomic layer deposition: An overview[J]. Chem Rev, 2009, 110 (1): 111-131. doi: 10.1021/cr900056b [18] GAO Z, DONG M, WANG G Z, SHENG P, WU Z W, YANG H M, ZHANG B, WANG G F, WANG J G, QIN Y. Multiply confined nickel nanocatalysts produced by atomic layer deposition for hydrogenation reactions[J]. Angew Chem Int Ed, 2015, 54 (31): 9006-9010. doi: 10.1002/anie.201503749 [19] LU J L, ELAM J W, STAIR P C. Atomic layer deposition-Sequential self-limiting surface reactions for advanced catalyst "bottom-up" synthesis[J]. Surf Sci Rep, 2016, 71 (2): 410-472. doi: 10.1016/j.surfrep.2016.03.003 [20] LI J W, ZHANG B, CHEN Y, ZHANG J K, YANG H M, ZHANG J W, LU X L, LI G C, QIN Y. Styrene hydrogenation performance of Pt nanoparticles with controlled size prepared by atomic layer deposition[J]. Catal Sci Technol, 2015, 5 (8): 4218-4223. doi: 10.1039/C5CY00598A [21] ZHANG B, CHEN Y, LI J W, PIPPEL E, YANG H M, GAO Z, QIN Y. High efficiency Cu-ZnO hydrogenation catalyst: The tailoring of Cu-ZnO interface sites by molecular layer deposition[J]. ACS Catal, 2015, 5 (9): 5567-5573. doi: 10.1021/acscatal.5b01266 [22] ZHAN B, GUO X W, LIANG H J, GE H B, GU X M, CHEN S, YANG H M, QIN Y. Tailoring Pt-Fe2O3 interfaces for selective reductive coupling reaction to synthesize imine[J]. ACS Catal, 2016, 6 (10): 6560-6566. doi: 10.1021/acscatal.6b01756 [23] WANG M H, GAO Z, ZHANG B, YANG H M, QIAO Y, CHEN S, GE H B, ZHANG J K, QIN Y. Ultrathin coating of confined Pt nanocatalysts by atomic layer deposition for enhanced catalytic performance in hydrogenation reactions[J]. Chem Eur J, 2016, 22 (25): 8438-8443. doi: 10.1002/chem.v22.25 [24] SUN S H, ZHANG G X, GAUQUELIN N, CHEN N, ZHOU J Q, YANG S Y, CHEN W F, MENG X B, GENG D S, BANIS M N, LI R Y, YE S Y, KNIGHTS S, BOTTON G A, SHAM T K, SUN X. Single-atom catalysis using Pt/graphene achieved through atomic layer deposition[J]. Sci Rep, 2013, 3 : 1775. doi: 10.1038/srep01775 [25] DENDOOVEN J, DEVLOO-CASIER K, IDE M, GRANDFIELD K, DETAVERNIER C. Atomic layer deposition-based tuning of the pore size in mesoporous thin films studied by in situ grazing incidence small angle X-ray scattering[J]. Nanoscale, 2014, 6 (24): 14991-14998. doi: 10.1039/C4NR05049E [26] DETAVERNIER C, DENDOOVEN J, SREE S P, LUDWIG K F, MARTENS J A. Tailoring nanoporous materials by atomic layer deposition[J]. Chem Soc Rev, 2011, 40 (11): 5242-5253. doi: 10.1039/c1cs15091j [27] HE Y J, NIVARTHY G S, EDER F, SESHAN K, LERCHER J A. Synthesis, characterization and catalytic activity of the pillared molecular sieve MCM-36[J]. Microporous Mesoporous Mater, 1998, 25 (1): 207-224. http://doc-test.utsp.utwente.nl/73860/ [28] ARICOÁA A S, SHUKLAB A K, KIMC H, PARKC S, MINC M, ANTONUCCIA V. An XPS study on oxidation states of Pt and its alloys with Co and Cr and its relevance to electroreduction of oxygen[J]. Appl Surf Sci, 2001, 172 (1): 33-40. http://cat.inist.fr/?aModele=afficheN&cpsidt=893464 [29] LEI Y, LU J L, LUO X Y, WU T P, DU P, ZHANG X Y, REN Y, WEN J G, MILLER D J, MILLER J T, SUN Y K, ELAM J W, AMINE K. Synthesis of porous carbon supported palladium nanoparticle catalysts by atomic layer deposition: Application for rechargeable lithium-O2 battery[J]. Nano Lett, 2013, 13 (9): 4182-4189. doi: 10.1021/nl401833p -





 下载:
下载: