Study on the mercury removal using Mn loaded Fe-based MOFs
-
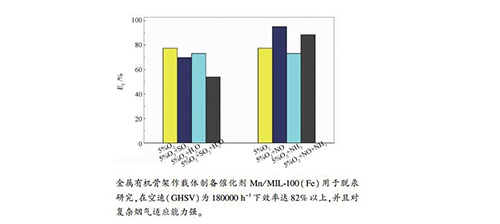
摘要: 基于Fe基金属有机骨架(MOFS)作为载体,采用浸渍法制备了负载6% Mn的Mn/MIL-100(Fe)脱汞剂。在模拟烟气中,搭建固定床研究了Mn/MIF-100(Fe)脱除单质汞(Hg0)性能。采用X射线衍射分析(XRD)、X射线电子能谱(XPS)、N2吸附-脱附(BET)和热重分析(TGA)对材料进行表征。研究表明,Mn/MIF-100(Fe)脱除单质汞(Hg0)效率较高,在250℃,空速(GHSV)为180000h-1时,脱汞(Hg0)效率达82%以上。Mn/MIF-100(Fe)主要的脱汞机理是催化氧化,Mn的负载促进了汞的吸附,并随着烟气温度的提高,单质汞的氧化效率逐渐提高。O2和NO促进汞的脱除,SO2和NH3抑制汞的脱除。Mn/MIL-100(Fe)整体上对复杂烟气的适应能力强。Abstract: The Mn/MIL-100 (Fe) mercury removal agent loaded with 6% Mn was prepared by impregnation method using Fe-based metal organic framework (MOFs) as the support. A set of fixed bed reactor apparatus was installed and used to study the performance of Mn/MIF-100(Fe) to remove elemental mercury (Hg0) in the simulated flue gas. The materials were characterized by XRD, XPS, BET and TGA. The results showed that Mn/MIF-100 (Fe) had high efficiency in removing elemental mercury (Hg0). When the GHSV was 180000 h-1 at 250℃, the mercury removal (Hg0) efficiency was above 82%. The main mercury removal mechanism of Mn/MIF-100 (Fe) was oxidation, and the loading of Mn promoted the adsorption of mercury. With the increase of flue gas temperature, the oxidation efficiency of elemental mercury was gradually increased. O2 and NO promoted the removal of mercury while SO2 and NH3 inhibited the removal of mercury. Mn/MIL-100(Fe) had a strong adaptability to the complex flue gas as a whole.
-
Key words:
- Fe based MOFs /
- adsorbent for mercury /
- adsorption /
- oxidation
-
表 1 脱汞剂的比表面积、孔容和孔径
Table 1 Specific surface area, pore volume and pore diameter ofthe catalysts
Sample BET surface area A/(cm2·g-1) Pore volume v/(cm3·g-1) Average pore diameter d/nm MIL-100(Fe) 1223.3 0.66 2.17 Mn/MIL-100(Fe) 141.8 0.29 8.31 Mn/Fe2O3 7.6 0.0007 0.04 表 2 催化剂金属离子各价态变换
Table 2 Percentage of valence state of each ion in the catalysts
Catalyst Percentagew/% Mn2+/Mnx+ Mn3+/ Mnx+ Mn4+/Mnx+ Fe2+/ Fex+ Fe3+/Fex+ OⅠ/(OⅠ+ OⅡ) OⅡ/(OⅠ+ OⅡ) Mn/ MIL-100(Fe) fresh
used6.12
6.2467.72
63.7626.16
3042.2
39.857.80
60.0377.44
67.7322.56
32.27Mn/Fe2O3 fresh
used-
-32.34
50.6067.66
49.4036.34
39.9763.36
60.0362.02
60.2837.98
39.71Mnx+: Mn2++Mn3++Mn4+; Fex+: Fe2++Fe3+ -
[1] DAHLAN I, KEATTEONG L, KAMARUDDIN A H, MOHAMED A R. Selection of metal oxides in the preparation of rice husk ash (RHA)/CaO sorbent for simultaneous SO2 and NO removal[J]. J Hazard Mater, 2009, 166(2):1556-1559. http://www.sciencedirect.com/science/article/pii/S0304389408018645 [2] LI H L, WU C Y, LI Y, ZHANG J Y. CeO2-TiO2 catalysts for catalytic oxidation of elemental mercury in low-rank coal combustion flue gas[J]. Environ Sci Technol, 2011, 45(17):7394-7400. doi: 10.1021/es2007808 [3] YUAN Y, ZHANG J Y, LI H L, LI Y, ZHAO Y C, ZHENG C G. Simultaneous removal of SO2, NO and mercury using TiO2-aluminum silicate fiber by photocatalysis[J]. Chem Eng J, 2012, 192(2):21-28. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=9e0e9f1bc91a9285b455b29f88706dbf [4] LI Y, MURPHY P D, WU C Y, POWERS K W, BONZONGO J-C J. Development of silica/vanadia/titania catalysts for removal of elemental mercury from coal-combustion flue gas[J]. Environ Sci Technol, 2008, 42(14):5304-5309. doi: 10.1021/es8000272 [5] LI B, WANG H L, XU Y Y, XUE J M. Study on mercury oxidation by SCR catalyst in coal-fired power plant[J]. Chem Eng J, 2017, 141(3):339-344. http://www.onacademic.com/detail/journal_1000040163327810_21ba.html [6] ESWARAN S, STENGER H G. Understanding mercury conversion in selective catalytic reduction (SCR) catalysts[J]. Energy Fuels, 2005, 19(6):2328-2334. doi: 10.1021/ef050087f [7] LI H L, WU C Y, LI Y, ZHANG J Y. Superior activity of MnOx-CeO2/TiO2 catalyst for catalytic oxidation of elemental mercury at low flue gas temperatures[J]. Appl Catal B:Environ, 2012, 111-112(3):381-388. http://www.sciencedirect.com/science/article/pii/S0926337311004917 [8] LIU Y, WANG Y J, WANG H Q, WU Z B. Catalytic oxidation of gas-phase mercury over Co/TiO2 catalysts prepared by sol-gel method[J]. Catal Commun, 2011, 12(14):1291-1294. doi: 10.1016/j.catcom.2011.04.017 [9] YAMAGUCHI A, AKIHO H, ITO S. Mercury oxidation by copper oxides in combustion flue gases[J]. Powder Technol, 2008, 180(1/2):222-226. doi: 10.1016-j.powtec.2007.03.030/ [10] ZHANG F, CHEN C, XIAO W M, XU LI, ZHANG N. CuO/CeO2 catalysts with well-dispersed active sites prepared from Cu3(BTC)2 metal-organic framework precursor for preferential CO oxidation[J]. Catal Commun, 2012, 26(35):25-29. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=77699f83a16ad842cf231c8776d0ab99 [11] ROSI N L, ECKERT J, EDDAOUDI M, VODAK D T, KIM J, O'KEEFFE M, YAGHI O M. Hydrogen storage in microporous metal-organic frameworks[J]. Science, 2003, 300(5622):1127-1129. doi: 10.1126/science.1083440 [12] YAN B L, ZHANG L J, TANG Z Y, AL-MAMUN M, ZHAO H J, SU X T. Palladium-decorated hierarchical titania constructed from the metal-organic frameworks NH2 -MIL-125(Ti) as a robust photocatalyst for hydrogen evolution[J]. Appl Catal B:Environ, 2017, 218(5):743-750. [13] LU W G, WEI Z W, GU Z Y, LIU T F, PARK J, PARK J, TIAN J, ZHANG M W, ZHANG Q, THOMAS G, BOSCH M, ZHOU H. Tuning the structure and function of metal-organic frameworks via linker design[J]. Chem Soc Rev, 2014, 43(16):5561-5593. doi: 10.1039/C4CS00003J [14] XU B, LI X J, CHEN Z M, ZHANG T, LI C C. Pd@MIL-100(Fe) composite nanoparticles as efficient catalyst for reduction of 2/3/4-nitrophenol:Synergistic effect between Pd and MIL-100(Fe)[J]. Microporous Mesoporous Mater, 2018, 255(1):1-6. http://cn.bing.com/academic/profile?id=cfe88f51a3465546bd25b4301d378a99&encoded=0&v=paper_preview&mkt=zh-cn [15] BHATTACHARJEE A, GUMMA S, PURKAIT M K. Fe3O4 promoted metal organic framework MIL-100(Fe) for the controlled release of doxorubicin hydrochloride[J]. Microporous Mesoporous Mater, 2017, 259(15):203-210. [16] ZHANG X, SHEN B X, ZHANG X Q, WANG F M, CHI G L, SI M. A comparative study of manganese-cerium doped metal-organic frameworks prepared via impregnation and in situ methods in the selective catalytic reduction of NO[J]. RSC Adv, 2017, 7(10):5928-5936. doi: 10.1039/C6RA25413F [17] YANG J P, ZHAO Y C, LIANG S F, ZHANG S B, MA S M, LI H L, ZHANG J Y, ZHENG C G. Magnetic iron-manganese binary oxide supported on carbon nanofiber (Fe3-x MnxO4/CNF) for efficient removal of Hg0 from coal combustion flue gas[J]. Chem Eng J, 2018, 334(15):216-224. [18] ZHANG S B, ZHAO Y C, YANG J P, ZHANG J Y, ZHENG C G. Fe-modified MnOx/TiO2 as the SCR catalyst for simultaneous removal of NO and mercury from coal combustion flue gas[J]. Chem Eng J, 2018, 348(15):618-629. [19] SHEN B X, WANG Y Y, WANG F M, LIU T. The effect of Ce-Zr on NH3-SCR activity over MnOx (0.6)/Ce0.5Zr0.5O2 at low temperature[J]. Chem Eng J, 2014, 236(2):171-180. http://www.sciencedirect.com/science/article/pii/S1385894713012783 [20] XU Y L, ZHONG Q, LIU X Y. Elemental mercury oxidation and adsorption on magnesite powder modified by Mn at low temperature[J]. J Hazard Mater, 2015, 283(11):252-259. http://www.ncbi.nlm.nih.gov/pubmed/25282177 [21] LEE C W, SERRE S D, ZHAO Y X, LEE S J, HASTINGS T W. Mercury oxidation promoted by a selective catalytic reduction catalyst under simulated Powder River Basin coal combustion conditions[J]. J Air Waste Manage, 2008, 58(4):484-493. doi: 10.3155/1047-3289.58.4.484 [22] LI H L, YING L, WU C Y, ZHANG J Y. Oxidation and capture of elemental mercury over SiO2-TiO2-V2O5 catalysts in simulated low-rank coal combustion flue gas[J]. Chem Eng J, 2011, 169(1):186-193. http://www.sciencedirect.com/science/article/pii/S1385894711002890 [23] RODRIGUEZ J A, HRBEK J. Interaction of sulfur with well-defined metal and oxide surfaces:Unraveling the mysteries behind catalyst poisoning and desulfurization[J]. Accounts Chem Res, 1999, 32(9):719-728. doi: 10.1021/ar9801191 [24] ZHANG X, SHEN B X, ZHU S W, XU H, TIAN L H. UiO-66 and its Br-modified derivates for elemental mercury removal[J].J Hazard Mater, 2016, 320(15):556-563. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=e1dd9a40a7a6b457343bcaa432976a72 [25] LEE J G, JOSHI B N, SAMUEL E, AN S, SWIHART M T, LEE J S, HWANG Y K, CHANG J S, YOON S S. Supersonically sprayed gas-and water-sensing MIL-100(Fe) films[J]. J Alloy Compd, 2017, 722(25):996-1001. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=095ff542beba20f29bb2dc64816f7ec4 [26] LI Y, MURPHY P D, WU C Y, POWERS K W, BONZONGO J C. Development of silica/vanadia/titania catalysts for removal of elemental mercury from coal-combustion flue gas[J]. Environ Sci Technol, 2008, 42(14):5304-5309. doi: 10.1021/es8000272 [27] LI H L, WU C Y, LI Y, ZHANG J Y. Superior activity of MnOx -CeO2/TiO2 catalyst for catalytic oxidation of elemental mercury at low flue gas temperatures[J]. Appl Catal B:Environ, 2012, s111-112(3):381-388. [28] XU H M, ZAN Q, ZONG C X, QUAN F Q, JIAN M, YAN N Q.Catalytic oxidation and adsorption of Hg0 over low-temperature NH3 -SCR LaMnO3 perovskite oxide from flue gas[J]. Appl Catal B:Environ, 2016, 186(5):30-40. http://www.sciencedirect.com/science/article/pii/S092633731530326X -





 下载:
下载: