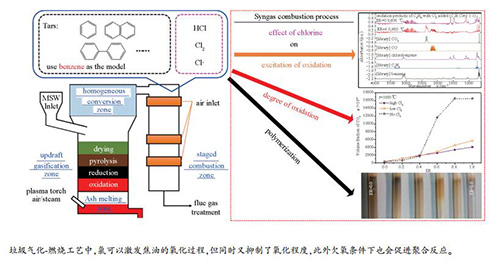
| [1] |
陈彤.城市生活垃圾焚烧过程中二噁英的形成机理及控制技术研究[D].杭州: 浙江大学, 2006.CHEN Tong. Formation mechanism and inhibition technologies of PCDD/Fs produced from MSW incineration[D]. Hangzhou: Zhejiang University, 2006.
|
| [2] |
张向和.垃圾处理场的邻避效应及其社会冲突解决机制的研究[D].重庆: 重庆大学, 2010.ZHANG Xiang-he. NIMBY of waste treatment plant and its solution mechanism of social conflict[D]. Chongqing: Chongqing University, 2010.
|
| [3] |
PÉREZ-RAMÍREZ J, MONDELLI C, SCHMIDT T, SCHLVTER O F K, WOLF A, MLECZKO L, DREIER T. Sustainable chlorine recycling via catalysed HCl oxidation:from fundamentals to implementation[J]. Energy Environ Sci, 2011, 4(12):4786-4799. doi: 10.1039/c1ee02190g
|
| [4] |
陆胜勇.垃圾和煤燃烧过程中二噁英的生成、排放和控制机理研究[D].杭州: 浙江大学, 2004.LU Sheng-yong. Dioxin formation inhibition and control mechanisms from coal and waste combustion processes[D]. Hangzhou: Zhejiang University, 2004.
|
| [5] |
ALTARAWNEH M, DLUGOGORSKI B Z, KENNEDY E M, MACKIE J C. Mechanisms for formation, chlorination, dechlorination and destruction of polychlorinated dibenzo-dioxins and dibenzofurans (PCDD/Fs)[J]. Prog Energy Combust, 2009, 35(3):245-274. doi: 10.1016/j.pecs.2008.12.001
|
| [6] |
PANEPINTO D, TEDESCO V, BRIZIO E, GENON G. Environmental performances and energy efficiency for MSW gasification treatment[J]. Waste Biomass Valorization, 2015, 6(1):123-135. doi: 10.1007/s12649-014-9322-7
|
| [7] |
ZHANG R Z, YIN R H, LUO Y H. Elimination of C-Cl bonds by a homogeneous conversion with sufficient H2:An attempt for the control of dioxin emission in Municipal Solid Waste gasification[J]. Energy Fuels, 2015, 27(9):4453-4462. https://es.scribd.com/doc/27076156/Waste-Incineration-Bref-0806
|
| [8] |
YIN R H, ZHANG R Z, LUO Y H. The effect of utilizing homogeneous conversion to control the formation of chlorinated hydrocarbons during PVC pyrolysis[J]. Environ Prog Sustainable Energy, 2016, 35(4):1012-1019. doi: 10.1002/ep.12313
|
| [9] |
张睿智, 罗永浩, 殷仁豪.垃圾气化过程中H2对二噁英抑制作用的实验研究[J].中国电机工程学报, 2016, 36(8):2195-2201. http://cdmd.cnki.com.cn/Article/CDMD-10248-1016787428.htmZHANG Rui-zhi, LUO Yong-hao, YIN Ren-hao. Experimental study on the inhibition effect of H2 on dioxin emission in MSW gasification[J]. Proc CSEE, 2016, 36(8):2195-2201. http://cdmd.cnki.com.cn/Article/CDMD-10248-1016787428.htm
|
| [10] |
WIKSTRÖM E, RYAN S, TOUATI A, TELFER M, TABOR D, GULLETT B K. Importance of chlorine speciation on de novo formation of polychlorinated dibenzo-p-dioxins and polychlorinated dibenzofurans[J]. Environ Sci Technol, 2003, 37(6):1108-1113. doi: 10.1021/es026262d
|
| [11] |
GULLETT B K, SAROFIM A F, SMITH K A, PROCACCINI C. The role of chlorine in dioxin formation[J]. Process Saf Environ, 2000, 78(1):47-52. doi: 10.1205/095758200530448
|
| [12] |
BASU P. Biomass Gasification and Pyrolysis:Practical Design and Theory[M]. USA:Academic press, 2010.
|
| [13] |
PROCACCINI C, BOZZELLI J W, LONGWELL J P, SAROFIM A F, SMITH K A. Formation of chlorinated aromatics by reactions of Cl·, Cl2, and HCl with benzene in the cool-down zone of a combustor[J]. Environ Sci Technol, 2003, 37(8):1684-1689. doi: 10.1021/es011432s
|
| [14] |
王波, 池涌, 严建华, 倪明江.氯对CO氧化抑制作用的实验研究[J].中国电机工程学报, 2009, 29(2):58-62. doi: 10.3321/j.issn:0258-8013.2009.02.011WANG Bo, CHI Yong, YAN Jian-hua, NI Ming-jiang. Experimental study on CO oxidation inhibited by chlorine[J]. Proc CSEE, 2009, 29(2):58-62. doi: 10.3321/j.issn:0258-8013.2009.02.011
|
| [15] |
ROESLER J R, YETTER R A, DRYER F L. Detailed kinetic modeling of moist CO oxidation inhibited by trace quantities of HCl[J]. Combust Sci Technol, 1992, 85(1/6):1-22. doi: 10.1080-00102209208947157/
|
| [16] |
TAYLOR P H, LENOIR D. Chloroaromatic formation in incineration processes[J]. Sci Total Environ, 2001, 269(1):1-24. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=JJ027755886
|





 下载:
下载: