Production of liquid bio-fuel from catalytic de-oxygenation: Pyrolysis of beech wood and flax shives
-
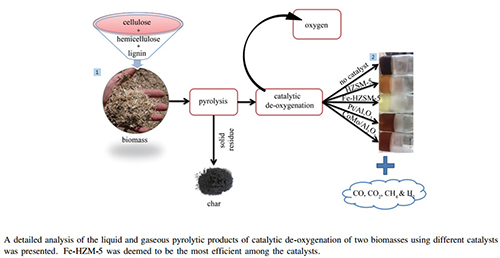
Abstract: This study presents a detailed analysis of the catalytic de-oxygenation of the liquid and gaseous pyrolytic products of two biomasses (beech wood and flax shives) using different catalysts (commercial HZSM-5 and H-Y, and lab-synthesised Fe-HZSM-5, Fe-H-Y, Pt/Al2O3 and CoMo/Al2O3). The experiments were all conducted in a semi-batch reactor under the same operating conditions for all feed materials. BET specific surface area, BJH pore size distribution and FT-IR technologies have been used to characterise the catalysts, while gas chromatography-mass spectrometry (GC-MS), flame ionisation detection (GC-FID) and thermal conductivity detection (GC-TCD) were used to examine the liquid and gaseous pyrolytic products. It was firstly seen that at higher catalyst-to-biomass ratios of 4:1, de-oxygenation efficiency did not experience any further significant improvement. Fe-HZSM-5 was deemed to be the most efficient of the catalysts utilised as it helped reach the lowest oxygen contents in the bio-oils samples and the second best was HZSM-5. It was also found that HZSM-5 and H-Y tended to privilege the decarbonylation route (production of CO), whilst their iron-modified counterparts favoured the decarboxylation one (production of CO2) for both biomasses studied. It was then seen that the major bio-oil components (carboxylic acids) underwent almost complete conversion under catalytic treatment to produce mostly unoxygenated aromatic compounds, phenols and gases like CO and CO2. Finally, phenols were seen to be the family most significantly formed from the actions of all catalysts.
-
Key words:
- pyrolysis /
- biomass /
- catalytic treatment /
- de-oxygenation /
- bio-oil upgrading
-
Table 1 Ultimate analysis of the biomasses
Biomass Ultimate analysis w/% carbon hydrogen nitrogen oxygen Flax shives (FS) 45.70 5.77 0.41 48.12 Beech wood (BW) 47.38 6.11 < 0.01 46.51 Table 2 Proximate analysis of biomasses based on TGA experiments
Biomass Proximate analysis w/% M V FC A Flax shives (FS) 2.78 74.72 19.97 2.53 Beech wood (BW) 5.70 75.93 17.52 0.85 Table 3 Specific surface areas and specific pore volumes of catalysts used
Catalyst used HZSM-5 Fe-HZSM-5 H-Y Fe-H-Y Al2O3 Pt/Al2O3 CoMo/Al2O3 Specific surface area A/(m2·g-1) 285.7 220.8 763.3 457.7 179.6 30.7 33.3 Specific pore volume v/(cm3·g-1) 0.41 0.25 0.48 - 0.22 - - Table 4 Parameters for experimental runs concerning effect of catalyst-to-biomass ratio used
Mass of Fe-HZSM-5 used m/g Height of Fe-HZSM-5 bed/cm Volume of catalytic zone without presence of catalyst v/cm3 Catalyst-to-biomass ratio used Contact t/s 6 2.5 26.55 2: 1 1.94 12 5.0 53.09 4: 1 3.89 28 10.0 106.19 9: 1 7.77 Table 5 Evolution of percentage of chemical families present in bio-oil from flax shives samples with different catalyst-to-biomass ratios
Chemical family present in bio-oil Bio-oil samples w/% catalyst : biomass ratio 0 2 4 9 Carboxylic acids 34.41 18.79 - - Alkanes 1.86 1.29 - - Aromatics 4.95 6.00 10.30 17.00 Alcohols 10.18 5.82 - - Aldehydes 3.07 1.78 3.26 7.36 Amides 15.13 - - - Ketones 6.93 11.73 12.44 20.96 Esters 9.57 4.03 - - Furans 0.70 1.74 - - Guaiacols 1.20 2.18 13.26 10.72 Phenols 8.31 46.65 60.74 43.96 Carbohydrates 3.68 - - - Table 6 Percentages of chemical families present in bio-oil samples with and without catalytic treatment
Percentage wmol/% beech wood bio-oils flax shive bio-oils no catalyst HZSM-5 Fe-HZSM-5 H-Y Fe-H-Y Pt/
Al2O3CoMo/
Al2O3no catalyst HZSM-5 Fe-HZSM-5 H-Y Fe-H-Y Pt/
Al2O3CoMo/
Al2O3Carboxylic acids 36.36 7.50 - 19.83 13.99 23.52 35.31 37.26 - - 30.78 7.83 33.62 36.25 Alkanes 0.85 - - - - 2.91 2.19 2.01 - - - - 2.51 1.38 Aromatics 4.83 13.28 8.97 8.07 4.89 7.77 5.18 4.54 12.15 10.51 7.11 5.26 5.19 4.13 Alcohols 7.36 7.47 5.09 8.61 5.83 12.28 8.88 11.02 9.27 5.79 9.25 5.37 13.52 7.29 Aldehydes 3.62 1.75 1.04 1.87 1.31 4.29 1.81 3.33 0.57 0.82 1.46 2.44 1.84 0.43 Amides 3.92 3.82 - 5.30 2.27 2.36 1.25 3.48 3.22 1.36 3.74 2.24 2.09 0.50 Ketones 10.26 6.21 7.30 5.29 3.79 9.69 10.37 8.53 5.35 9.04 3.84 3.52 15.26 14.53 Esters 9.58 5.42 - 11.41 3.53 10.83 5.17 10.85 1.49 - 11.35 1.89 6.55 3.87 Furans 2.16 4.56 3.99 3.08 2.74 1.03 2.19 0.76 3.04 5.64 2.65 2.54 1.18 1.89 Guaiacols 1.34 3.06 2.07 4.10 1.44 0.91 0.88 1.30 1.05 1.66 3.21 1.39 - 1.06 Phenols 14.46 46.92 71.53 32.43 60.22 16.96 22.05 12.92 63.86 65.18 26.62 67.54 12.54 23.86 Carbohydrates 5.26 - - - - 7.45 4.72 3.99 - - - - 5.70 4.80 Table 7 Percentages of gaseous components present in non-condensable gas samples with and without catalytic treatment
Percentage φ/% beech wood non-condensable gases flax shive non-condensable gases no catalyst HZSM-5 Fe-HZSM-5 Pt/
Al2O3CoMo/
Al2O3H-Y Fe-H-Y no catalyst HZSM-5 Fe-HZSM-5 Pt/
Al2O3CoMo/
Al2O3H-Y Fe-H-Y H2 1.04 0.93 15.28 36.23 6.97 1.13 10.10 1.30 1.35 13.77 31.91 6.91 1.42 10.03 CO 44.61 49.60 28.61 24.67 42.22 52.38 35.52 35.39 42.34 25.83 25.41 33.46 42.87 28.38 CO2 39.99 32.36 38.61 29.73 37.08 28.67 34.46 50.18 35.79 43.41 32.10 44.91 38.79 41.59 CH4 11.38 5.26 6.18 8.49 11.04 11.87 14.78 10.51 7.29 6.31 8.34 10.24 11.50 14.17 C2H4 1.64 6.66 5.10 0.39 1.47 3.04 2.31 1.40 6.81 4.92 0.66 1.43 2.86 2.13 C2H6 1.13 0.46 1.46 0.48 0.83 1.09 0.94 1.20 0.85 0.65 0.66 1.05 1.40 1.11 C3H6 - 4.73 4.74 - 0.04 1.81 1.88 - 5.56 4.81 0.64 1.36 0.64 1.80 Table 8 Water content of bio-oil samples
Biomass Catalyst used Water content w/% Standard error /% Beech wood no catalyst 2.39 0.14 HZSM-5 6.06 0.06 Fe-HZSM-5 5.45 0.10 H-Y 3.81 0.05 Fe-H-Y 2.63 0.26 Pt/Al2O3 3.33 0.01 CoMo/Al2O3 4.65 0.33 Flax shive no catalyst 1.23 0.04 HZSM-5 5.45 0.23 Fe-HZSM-5 5.08 0.07 H-Y 3.92 0.08 Fe-H-Y 2.73 0.18 Pt/Al2O3 1.86 0.01 CoMo/Al2O3 3.76 0.29 Table 9 Conversion and production rates of chemical families present in bio-oil samples obtained with and without catalyst use
Chemical families Conversion ("-" sign) and production ("+" sign) rate /% beech wood bio-oil flax shive bio-oil HZSM-5 Fe-HZSM-5 H-Y Fe-H-Y Pt/
Al2O3CoMo/
Al2O3HZSM-5 Fe-HZSM-5 H-Y Fe-H-Y Pt/
Al2O3CoMo/
Al2O3Carboxylic acids -84 -100 -69 -84 -53 -60 -100 -100 -55 -83 -53 -67 Alkanes -100 -100 -100 -100 +37 +223 -100 -100 -100 -100 +40 +291 Aromatics +372 +23 +159 +4 +217 +74 +133 +30 +60 +11 +122 +27 Alcohols -27 -83 -39 -70 +11 +48 N.C. -70 +1 -61 +37 +83 Aldehydes -65 -93 -73 -86 -20 -93 -80 -86 -47 -41 -38 +113 Amides -71 -100 -48 -95 -46 -29 -67 -95 -39 -89 -67 -98 Ketones -45 -78 -66 -82 -33 -65 -15 -31 -38 -62 +128 +60 Esters -61 -100 -40 -86 -31 -4 -83 -100 -46 -85 -29 +19 Furans +322 +27 +107 +35 -11 -329 +374 +324 +319 +168 +73 +734 Guaiacols +82 -58 +76 -55 -50 +168 -3 -27 +199 -14 -100 +289 Phenols +267 +92 +65 +151 +4 -24 +766 +305 +257 +505 +56 N.C. Carbohydrates -100 -100 -100 -100 -20 -92 -100 -100 -100 -100 -41 -46 conversion ratio = (moles obtained without de-oxygenation-moles obtained after de-oxygenation)/moles obtained without de-oxygenation × 100%
N.C.: no change (same as amount present in non-catalytic sample)Table 10 Conversion and production rates of non-condensable gas (NCG) components obtained with and without catalyst use
NCG component Conversion ("-" sign) and production ("+" sign) rate /% beech wood NCG flax shives NCG HZSM-5 Fe-HZSM-5 H-Y Fe-H-Y Pt/
Al2O3CoMo/
Al2O3HZSM-5 Fe-HZSM-5 H-Y Fe-H-Y Pt/
Al2O3CoMo/
Al2O3H2 +67 +4511 +116 +2565 +13960 +1173 +101 +2774 +79 +1837 +8199 +886 CO +109 +101 +132 +118 +123 +79 +131 +97 +98 +101 +142 +75 CO2 +52 +202 +42 +136 +199 +76 +38 +134 +26 +107 +116 +65 CH4 -13 +70 +106 +256 +200 +84 +34 +62 +79 +237 +168 +80 C2H2 -100 -100 -100 -100 -100 -100 N.C. N.C. N.C. Prod. N.C. Prod. C2H4 +662 +874 +266 +286 -4 +70 +839 +849 +234 +280 +59 +88 C2H6 -23 +307 +92 +129 +71 +40 +37 +46 +90 +132 +85 +63 C3H4 Prod. N.C. Prod. Prod. Prod. Prod. -17 -19 +254 +2 +2 +121 C3H6 Prod. Prod. Prod. Prod. N.C. Prod. Prod. Prod. Prod. Prod. Prod. Prod. C3H8 N.C. N.C. N.C. N.C. N.C. Prod. N.C. Prod. Prod. Prod. Prod. Prod. conversion ratio = (moles obtained without de-oxygenation-moles obtained after de-oxygenation)/moles obtained without de-oxygenation × 100%
Prod.: production (produced because of the catalytic treatment, not present in non-catalytic sample);
N.C.: no change (same as amount present in non-catalytic sample) -
[1] Energetics Inc. Energy and Environmental Profile of the U.S[Z]. Chemical Industry. U.S. Department of Energy, Office of Industrial Technologies, 2000. [2] CHENG S, WEI L, ZHAO X, JULSON J. Application, deactivation, and regeneration of heterogeneous catalysts in bio-oil upgrading[J]. Catalysts, 2016, 6(12):195. doi: 10.3390/catal6120195 [3] GUDA V, TOGHIANI H. Catalytic upgrading of pinewood fast pyrolysis vapors using an integrated Auger-packed bed reactor system:Effects of acid catalysts on yields and distribution of pyrolysis products[J]. J Prod Ind, 2015, 4(2):33-43. [4] FRENCH R, CZERNIK S. Catalytic pyrolysis of biomass for biofuels production[J]. Fuel Process Technol, 2010, 91(1):25-32. http://www.sciencedirect.com/science/article/pii/S0378382009002392 [5] GAYUBO A G, AGUAYO A T, ATUTXA A, AGUADO R, BILBAO J. Transformation of oxygenate components of biomass pyrolysis oil on a HZSM-5 zeolite. I. Alcohols and phenols[J]. Ind Eng Chem Res, 2004, 43(11):2610-2618. doi: 10.1021/ie030791o [6] GUNAWARDENA D A, FERNANDO S D. Methods and applications of deoxygenation for the conversion of biomass to petrochemical products[C]//Biomass Now-Cultivation and Utilization. 2013. [7] ARENAMNART S, TRAKARNPRUK W. Ethanol conversion to ethylene using metal-mordenite catalysts[J]. Int J Appl Sci Eng, 2006, 4(1):21-32. [8] CHENG Y-T, JAE J, SHI J, FAN W, HUBER G W. Production of renewable aromatic compounds by catalytic fast pyrolysis of lignocellulosic biomass with bifunctional Ga/ZSM-5 catalysts[J]. Angew Chem, 2012, 124(6):1416-1419. doi: 10.1002/ange.201107390 [9] LI P, LI D, YANG H, WANG X, CHEN H. Effects of Fe-, Zr-, and Co-modified zeolites and pretreatments on catalytic upgrading of biomass fast pyrolysis vapors[J]. Energy Fuels, 2016, 30(4):3004-3013. doi: 10.1021/acs.energyfuels.5b02894 [10] MULLEN C A, BOATENG A A. Production of aromatic hydrocarbons via catalytic pyrolysis of biomass over Fe-modified HZSM-5 zeolites[J]. ACS Sustainable Chem Eng, 2015, 3(7):1623-1631. doi: 10.1021/acssuschemeng.5b00335 [11] SUN L, ZHANG X, CHEN L, ZHAO B, YANG S, XIE X. Comparision of catalytic fast pyrolysis of biomass to aromatic hydrocarbons over ZSM-5 and Fe/ZSM-5 catalysts[J]. J Anal Appl Pyrolysis, 2016, 121(Supplement C):342-346. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=9d9f0c7f1058285db99d90bb8d79565c [12] MORTENSEN P M, GRUNWALDT J-D, JENSEN P A, KNUDSEN K G, JENSEN A D. A review of catalytic upgrading of bio-oil to engine fuels[J]. Appl Catal A:Gen, 2011, 407(1/2):1-19. https://www.sciencedirect.com/science/article/pii/S0926860X11005138 [13] PAYORMHORM J, KANGVANSAICHOL K, REUBROYCHAROEN P, KUCHONTHARA P, HINCHIRANAN N. Pt/Al2O3-catalytic deoxygenation for upgrading of Leucaena leucocephala-pyrolysis oil[J]. Bioresour Technol, 2013, 139:128-135. doi: 10.1016/j.biortech.2013.04.023 [14] ZHANG J, WANG K, NOLTE M W, CHOI Y S, BROWN R C, SHANKS B H. Catalytic deoxygenation of bio-oil model compounds over acid-base bifunctional catalysts[J]. ACS Catal, 2016, 6(4):2608-2621. doi: 10.1021/acscatal.6b00245 [15] MOHABEER C, ABDELOUAHED L, MARCOTTE S, TAOUK B. Comparative analysis of pyrolytic liquid products of beech wood, flax shives and woody biomass components[J]. J Anal Appl Pyrolysis, 2017, 127:269-277. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=8f10dc0e1f0d3b42902b36a5dd5e9d7d [16] GARCÍA J R, BERTERO M, FALCO M, SEDRAN U. Catalytic cracking of bio-oils improved by the formation of mesopores by means of Y zeolite desilication[J]. Appl Catal A:Gen, 2015, 503:1-8. doi: 10.1016/j.apcata.2014.11.005 [17] AHO A, KUMAR N, LASHKUL A V, ERÄNEN K, ZIOLEK M, DECYK P, SALMI T, HOLMBOM B, HUPA M, MURZIN Y D. Catalytic upgrading of woody biomass derived pyrolysis vapours over iron modified zeolites in a dual-fluidized bed reactor[J]. Fuel, 2010, 89(8):1992-2000. doi: 10.1016/j.fuel.2010.02.009 [18] DUMEIGNIL F, SATO K, IMAMURA M, MATSUBAYASHI N, PAYEN E, SHIMADA H. Characterization and hydrodesulfurization activity of CoMo catalysts supported on sol-gel prepared Al2O3[J]. Appl Catal A:Gen, 2005, 287(1):135-145. doi: 10.1016/j.apcata.2005.03.034 [19] DEKA R C. Acidity in zeolites and their characterization by different spectroscopic methods[J]. Indian J Chem Technol, 1998, 5:109-123. [20] TOPALOǦLU Y D, BILGIÇ C. Determining the surface acidic properties of solid catalysts by amine titration using Hammett indicators and FTIR-pyridine adsorption methods[J]. Surf Interface Anal, 2010, 42(6/7):959-962. https://www.mendeley.com/catalogue/determining-surface-acidic-properties-solid-catalysts-amine-titration-using-hammett-indicators-ftirp/ [21] LI H, YAN Y, REN Z. Online upgrading of organic vapors from the fast pyrolysis of biomass[J]. J Fuel Chem Technol, 2008, 36(6):666-671. doi: 10.1016/S1872-5813(09)60002-5 [22] HORNUNG A. Intermediate Pyrolysis as an Alternative to Fast Pyrolysis[C]//Bioenergy Iv: Innovations in Biomass Conversion for Heat, Power, Fuels & Chemicals. 2014. http://dc.engconfintl.org/bioenergy_iv/2/ [23] MAHMOOD A S N, BRAMMER J G, HORNUNG A, STEELE A, POULSTON S. The intermediate pyrolysis and catalytic steam reforming of Brewers spent grain[J]. J Anal Appl Pyrolysis, 2013, 103:328-342. doi: 10.1016/j.jaap.2012.09.009 [24] TORRI I D, PAASIKALLIO V, FACCINI C S, HUFF R, CARAMÃO E B, SACON V, OASMAA A, ZINI C A. Bio-oil production of softwood and hardwood forest industry residues through fast and intermediate pyrolysis and its chromatographic characterization[J]. Bioresour Technol, 2016, 200:680-690. doi: 10.1016/j.biortech.2015.10.086 [25] KEBELMANN K, HORNUNG A, KARSTEN U, GRIFFITHS G. Intermediate pyrolysis and product identification by TGA and Py-GC/MS of green microalgae and their extracted protein and lipid components[J]. Biomass Bioenergy, 2013, 49:38-48. doi: 10.1016/j.biombioe.2012.12.006 [26] GARCÍA R, PIZARRO C, LAVÍN A G, BUENO J L. Biomass proximate analysis using thermogravimetry[J]. Bioresour Technol, 2013, 139:1-4. http://d.old.wanfangdata.com.cn/NSTLQK/NSTL_QKJJ0230160843/ [27] CHARON N, PONTHUS J, ESPINAT D, BROUST F. Multi-technique characterization of fast pyrolysis oils[J]. J Anal Appl Pyrolysis, 2015, 116:18-26. doi: 10.1016/j.jaap.2015.10.012 [28] JANNOT Y. Isothermes de sorption: Modèles et détermination[Z]. 2008. [29] WARD J W. Thermal decomposition of ammonium Y zeolite[J]. J Catal, 1970, 18(3):348-351. doi: 10.1016-0021-9517(70)90331-3/ [30] LOBREE L J, HWANG I-C, REIMER J A, BELL A T. Investigations of the state of Fe in H-ZSM-5[J]. J Catal, 1999, 186(2):242-253. https://www.sciencedirect.com/science/article/pii/S0021951799925484 [31] NAQVI S R, UEMURA Y, YUSUP S, SUGIUR Y, NISHIYAMA N, NAQVI M. The role of zeolite structure and acidity in catalytic deoxygenation of biomass pyrolysis vapors[J]. Energy Procedia, 2015, 75:793-800. doi: 10.1016/j.egypro.2015.07.126 [32] PUÉRTOLAS B, KELLER T C, MITCHELL S, PÉREZ-RAMÍREZ J. Deoxygenation of bio-oil over solid base catalysts:From model to realistic feeds[J]. Appl Catal B:Environ, 2016, 184:77-86. [33] GARCIA L, SALVADOR M L, ARAUZO J, BILBAO R. Influence of catalyst weight/biomass flow rate ratio on gas production in the catalytic pyrolysis of pine sawdust at low temperatures[J]. Ind Eng Chem Res, 1998, 37:3812-3819. [34] IMRAN A A, BRAMER E A, SESHAN K, BREM G. Catalytic flash pyrolysis of biomass using different types of zeolite and online vapor fractionation[J]. Energies, 2016, 9(3):187. doi: 10.3390/en9030187 [35] WANG C, HAO Q, LU D, JIA Q, LI G, XU B. Production of light aromatic hydrocarbons from biomass by catalytic pyrolysis[J]. Chin J Catal, 2008, 29(9):907-912. doi: 10.1016/S1872-2067(08)60073-X [36] YOO M L, PARK Y H, PARK Y-K, PARK S H. Catalytic pyrolysis of wild reed over a zeolite-based waste catalyst[J]. Energies, 2016, 9(3):201. doi: 10.3390/en9030201 [37] MUKARAKATE C, MCBRAYER J D, EVANS T, BUDHI S. Catalytic fast pyrolysis of biomass:the reactions of water and aromatic intermediates produces phenols[J]. Green Chem, 2015, 17(8):4217-4227. http://www.chemie.de/fachpublikationen/817857/catalytic-fast-pyrolysis-of-biomass-the-reactions-of-water-and-aromatic-intermediates-produces-phenols.html [38] GAYUBO A G, AGUAYO A T, ATUTXA A, AGUADO R, OLAZAR M, BILBAO J. Transformation of oxygenate components of biomass pyrolysis oil on a HZSM-5 Zeolite. Ⅱ. Aldehydes, ketones, and acids[J]. Ind Eng Chem Res, 2004, 43(11):2619-2626. https://www.mendeley.com/catalogue/transformation-oxygenate-components-biomass-pyrolysis-oil-hzsm5-zeolite-ii-aldehydes-ketones-acids/ [39] GUO Z, WANG S, ZHU Y, LUO Z, CEN K. Separation of acid compounds for refining biomass pyrolysis oil[J]. J Fuel Chem Technol, 2009, 37(1):49-52. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rlhxxb200901009 [40] YANG H, YAO J, CHEN G, MA W, YAN B, QI Y. Overview of upgrading of pyrolysis oil of biomass[J]. Energy Procedia, 2014, 61:1306-1309. doi: 10.1016/j.egypro.2014.11.1087 [41] YANG H, YAN R, CHEN H, LEE D H, ZHENG C. Characteristics of hemicellulose, cellulose and lignin pyrolysis[J]. Fuel, 2007, 86(12):1781-1788. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=3fa85712db16f40b571417e71fe15255 [42] CHANTAL P, KALIAGUINE S, GRANDMAISON J L, MAHAY A. Production of hydrocarbons from aspen poplar pyrolytic oils over H-ZSM5[J]. Appl Catal, 1984, 10(3):317-332. doi: 10.1016/0166-9834(84)80127-X -





 下载:
下载: