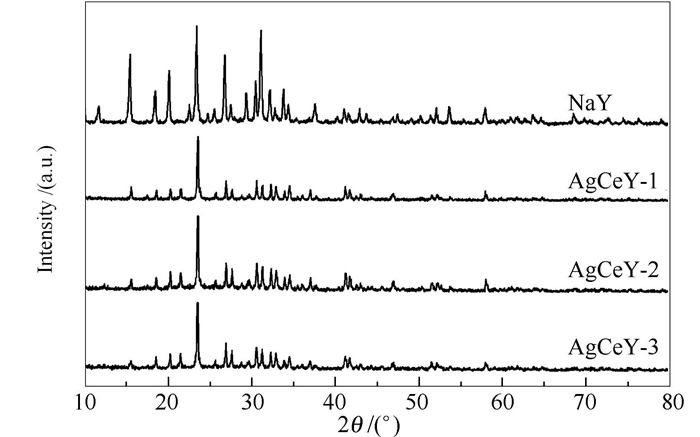
-
摘要: 以硅铝比为5.3的NaY分子筛为母体,分别采用微波辅助离子交换法(AgCeY-1)、水热离子交换法(AgCeY-2)和液相离子交换法(AgCeY-3)制备了AgCeY-n吸附剂,并利用XRD、BET、XPS和Py-FTIR对吸附剂进行了表征。以噻吩和苯并噻吩为模型硫化物,甲苯和环己烯为竞争吸附组分,考察了制备方法对制备得到的吸附剂脱硫性能的影响。结果表明,AgCeY-n吸附剂上Ag、Ce元素分别以Ag+、Ce4+形式存在。经微波辅助离子交换法制备得到的AgCeY-1吸附剂表面Ag+、Ce4+含量均最高,且具有最高的L酸和B酸量。AgCeY-n吸附剂对硫化物的吸附选择大小顺序为:BT>TP,竞争吸附组分对AgCeY-n吸附脱硫性能的影响顺序为:环己烯>甲苯。在所研究的制备方法中,微波辅助离子交换法所需时间最短(20 min),合成的AgCeY-1对所研究的模拟油的吸附效果均最好,且具有较好的重复使用性能。各吸附剂对TP和BT的脱除能力大小顺序为:AgCeY-1 >AgCeY-2 >AgCeY-3。Abstract: The AgCeY-n adsorbents were successfully synthesized by microwave-assisted liquid ion exchange (AgCeY-1), hydrothermal ion exchange (AgCeY-2) and liquid phase ion exchange (AgCeY-3) methods with NaY (Si/Al ration=5.3) molecular sieve parent and characterized by XRD, BET, XPS and Py-FTIR analysis. With thiophene (TP) and benzothiophene (BT) as the model sulfides, and toluene and cyclohexene as the competition components, the effects of the preparation method on the adsorptive desulfurization properties of the as-prepared adsorbents were evaluated. The results show that the Ag and Ce species in AgCeY-n zeolites exist as Ag+ and Ce4+. The AgCeY-1 prepared via microwave-assisted liquid ion exchange method possesses the highest Ag+ and Ce4+ contents. In addition, it possesses the highest amounts of L and B acid. The adsorption selectivity to sulfur compounds follows the order:BT>TP. The effects of competition components on the sulfur removal with AgCeY-n zeolites follow the order:cyclohexene>toluene. Among the preparation methods tested,the microwave-assisted liquid ion exchange method takes the shortest time (20 min). The as-prepared AgCeY-1 adsorbent exhibits most satisfied performance of adsorption for all the model oils and a wonderful regeneration property. The removal ability for TP and BT of various adsorbents follows the order:AgCeY-1 >AgCeY-2 >AgCeY-3.
-
Key words:
- AgCeY /
- microwave method /
- adsorptive desulfurization /
- regeneration /
- toluene /
- cyclohexene
-
表 1 各模拟油的组成
Table 1 The composition of the model fuels
No. Sulfur concentration c/(mg·L-1) Content c/(mg·L-1) TP BT toluene cyclohexene M1 100 100 - - M2 100 100 500 - M3 100 100 - 500 表 2 NaY和AgCeY-1、AgCeY-2和AgCeY-3吸附剂的结构性质
Table 2 Pore structure of NaY and AgCeY-n zeolites prepared by different methods
Sample Surface area
A/(m2·g-1)Pore volume
v/(cm3·g-1)Pore size
d/nmNaY 620 0.36 2.33 AgCeY-1 534 0.30 1.18 AgCeY-2 516 0.31 1.16 AgCeY-3 541 0.31 1.16 表 3 AgCeY-1、AgCeY-2和AgCeY-3 吸附剂的表面金属元素含量
Table 3 Metal content on the surface of AgCeY-1,AgCeY-2 and AgCeY-3
Sample Metal content w/% Ag Ce NaY - - AgCeY-1 1.87 1.24 AgCeY-2 1.57 1.17 AgCeY-3 1.54 1.07 表 4 吸附剂表面Lewis酸和Brønsted酸的分布
Table 4 The content distribution of Lewis and Brønsted acid
Sample Desorption
temperature t/℃Lewis acidity
/(μmol·g-1)Brønsted acidity
/(μmol·g-1)Total acidity
/(μmol·g-1)Lewis/total
acidityAgCeY-1 150 444.9 82.0 526.9 0.84 350 58.7 31.3 90.0 0.65 AgCeY-2 150 436.0 68.2 504.2 0.86 350 37.3 17.6 54.9 0.68 AgCeY-3 150 278.5 29.9 308.4 0.90 350 41.2 21.9 62.1 0.35 表 5 吸附模拟油M1,M2 和 M3后的AgCeY-1吸附剂再生性能
Table 5 The regeneration of the AgCeY-1 after absorption of model oil M1,M2 and M3
Times of
regenerationSulfur removal η /% M1 M2 M3 TP BT TP BT TP BT 0 99.9 100 84.8 99.9 80.1 99.8 1 99.6 100 82.2 99.7 76.6 99.4 2 90.1 99.9 79.4 99.0 74.4 98.6 3 98.3 99.1 76.6 98.0 72.1 97.5 4 97.0 98.6 72.2 96.2 68.2 96.6 5 95.0 98.0 66.6 94.0 62.3 94.1 -
[1] SONG H,CUI X H,SONG H L,GAO H J,LI F.Characteristic and adsorption desulfurization performance of Ag-Ce bimetal ion-exchanged Y zeolite[J].Ind Eng Chem Res,2014,53(37):14552-14557. doi: 10.1021/ie404362f [2] SONG C S.An overview of new approaches to deep desulfurization for ultra-clean gasoline,diesel fuel and jet fuel[J].Catal Today,2003,86:211-263. doi: 10.1016/S0920-5861(03)00412-7 [3] XIA Y T,LI Y K,GU Y T,JIN T,YANG Q,HU J,LIU H L,WANG H L.Adsorption desulfurization by hierarchical porous organic polymer of poly-methylbenzene with metal impregnation[J].Fuel,2016,170:100-106. doi: 10.1016/j.fuel.2015.12.047 [4] ZHAO Y W,SHEN B X,SUN H,ZHAN G X,LIU J C.Adsorption of dimethyl disulfide on ZSM-5 from methyl tert-butyl ether liquid:A study on equilibrium and kinetics[J].Fuel Process Technol,2016,145:14-19. doi: 10.1016/j.fuproc.2016.01.025 [5] SONG H,CHANG Y X,SONG H L.Deep adsorptive desulfurization over Cu,Ce bimetal ion-exchanged Y-typed molecule sieve[J].Adsorption,2016,22:139-150. doi: 10.1007/s10450-015-9731-3 [6] 周继红,罗一斌,宗保宁.Y型分子筛复合材料的成孔机理[J].石油炼制与化工,2012,43(1):26-31. http://www.cnki.com.cn/Article/CJFDTOTAL-SYLH201201012.htmZHOU Ji-hong,LUO Yi-bin,ZONG Bao-ning.Formation mechanism of porous molecular sieve Y composite[J].Pet Process Petrochem,2012,43(1):26-31. http://www.cnki.com.cn/Article/CJFDTOTAL-SYLH201201012.htm [7] HEMANDEZ-MALDONADO A J,YANG R T.Desulfurization of liquid fuels by adsorption viaπ-complexation with Cu (I)-Y and Ag-Y zeolites[J].Ind Eng Chem Res,2003,42(1):123-129. doi: 10.1021/ie020728j [8] OLIVEIRA M L M,MIRANDA A A L,BARBOSA C M B M,CACALCANTE C L,AZEVEDO D C S,RODRIGUEZ-CASTELLON E.Adsorption of thiophene and toluene on NaY zeolites exchanged with Ag (I),Ni (Ⅱ) and Zn (Ⅱ)[J].Fuel,2009,88(10):1885-1892. doi: 10.1016/j.fuel.2009.04.011 [9] SONG H,WAN X,SUN X.Preparation of AgY zeolites using microwave irradiation and study on their adsorptive desulphurisation performance[J].Can J Chem Eng,2013,91(5):915-923. doi: 10.1002/cjce.v91.5 [10] VELU S,MA X,SONG C.Selective adsorption for removing sulfur from jet fuel over zeolite-based adsorbents[J].Ind Eng Chem Res,2003,42(21):5293-5304. doi: 10.1021/ie020995p [11] SONG H,WAN X,DAI M,ZHANG J J,LI F,SONG H L.Deep desulfurization of model gasoline by selective adsorption over Cu-Ce bimetal ion-exchanged Y zeolite[J].Fuel Process Technol,2013,116:52-62. doi: 10.1016/j.fuproc.2013.04.017 [12] SONG H,CHANG Y X,WAN X,DAI M,SONG H L,JIN Z S.Equilibrium,kinetic and thermodynamic studies on adsorptive desulfurization onto CuICeIVY zeolite[J].Ind Eng Chem Res,2014,53:5701-5708. doi: 10.1021/ie403177t [13] 王娟,张海波,张秋卓,曾辉,蔡伟民.NiCeY改性沸石吸附燃料油中二苯并噻吩的研究[J].环境工程学报,2008,2(11):1581-1584. http://www.cnki.com.cn/Article/CJFDTOTAL-HJJZ200811031.htmWANG Juan,ZHANG Hai-bo,ZHANG Qiu-zhuo,ZENG Hui,CAI Wei-min.Study on adsorption of dibenzeothiophene in fuel using NiCeY zeolite[J].Chin J Environ Eng,2008,2(11):1581-1584. http://www.cnki.com.cn/Article/CJFDTOTAL-HJJZ200811031.htm [14] SONG H,GAO H J,SONG H L,YANG G,LI X J.Effects of Si/Al ratio of ion-exchanged AgCeY zeolites on the adsorption desulfurization[J].Ind Eng Chem Res,2016,55(13):3813-3822. doi: 10.1021/acs.iecr.5b04609 [15] 苑丹丹,宋华林,崔雪涵,高慧杰,宋华.AgCeY分子筛吸附剂的制备及性能研究[J].燃料化学学报,2015,43(5):620-627. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18632.shtmlYUAN Dan-dan,SONG Hua-lin,CUI Xue-han,GAO Hui-jie,SONG Hua.Study on preparation and characteristics of AgCeYzeolites[J].J Fuel Chem Technol,2015,43(5):620-627. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18632.shtml [16] 王晓晖,卜龙利,刘海楠,张浩,孙剑宇,杨力,蔡力栋.碳化硅协同分子筛负载型催化剂微波辅助催化氧化甲苯性能[J].环境科学学报,2013,34(6):2017-2116. http://www.cnki.com.cn/Article/CJFDTOTAL-HJKZ201306005.htmWANG Xiao-hui,BO Long-li,LIU Hai-nan,ZHANG Hao,SUN Jian-yu,YANG Li,CAI Li-dong.Synergetic effects of silicon carbide and molecular sieve loaded catalyst on microwave assisted catalytic oxidation of toluene[J].Acta Sci Circumstantiae,2013,34(6):2017-2116. http://www.cnki.com.cn/Article/CJFDTOTAL-HJKZ201306005.htm [17] 程志林,晁自胜,万惠霖.微波诱导快速合成纳米NaY分子筛[J].物理化学学报,2003,19(6):487-491. http://www.cnki.com.cn/Article/CJFDTOTAL-WLHX200306001.htmCHENG Zhi-lin,CHAO Zi-sheng,WAN Hui-lin.Nanosized NaY zeolite synthesized rapidly by microwave induction[J].Acta Phys Chim Sin,2003,19(6):487-491. http://www.cnki.com.cn/Article/CJFDTOTAL-WLHX200306001.htm [18] 李小娟.改性Y分子筛选择性吸附燃料油深度脱硫及等离子体再生技术研究[D].浙江:浙江大学,2009.LI Xiao-juan.Selective adsorption of thiophenic compounds from fuel over Y zeolite-based adsorbents and regeneration by non-thermal plasma processing[D].Zhejiang:Zhejiang Univesity,2009. [19] ROMEO M,BAK K,FALLAH J El,NORMAND L,HILAIREET L.XPS study of the reduction of cerium dioxide[J].Surf Interface Anal,1993,20(6):508-512. doi: 10.1002/(ISSN)1096-9918 [20] LI J C,ZENG P H,ZHAO L,REN S Y,GUO Q X,ZHAO H J,WANG B J,LIU H H,PANG X M,GAO X H,SHEN B J.Tuning of acidity in CeY catalytic cracking catalysts by controlling the migration of Ce in the ion exchange step through valence changes[J].J Catal,2015,329:441-448. doi: 10.1016/j.jcat.2015.06.012 [21] 邵红,霍超.微波技术在催化剂制备领域的应用研究[J].化工技术与开发,2006,35(11):2-5. http://www.cnki.com.cn/Article/CJFDTOTAL-GXHG200611000.htmSHAO Hong,HUO Chao.Application advances of microwave technology in preparation of catalysts[J].Technol Develop Chem Ind,2006,35(11):2-5. http://www.cnki.com.cn/Article/CJFDTOTAL-GXHG200611000.htm [22] TREJDA M,TUEL A,KUJAW J,KILOS B,ZIOLEK M.Niobium rich SBA-15 materials-preparation,characterisation and catalytic activity[J].Microporous Mesoporous Mater,2008,110:271-278. doi: 10.1016/j.micromeso.2007.06.015 [23] KALITA P,GUPTA N M,KUMAR R.Synergistic role of acid sites in the Ce-enhanced activity of mesoporous Ce-Al-MCM-41 catalysts in alkylation reactions:FTIR and TPD-ammonia studies[J].J Catal,2007,245:338-347. doi: 10.1016/j.jcat.2006.10.022 [24] GONG Y J,DOU T,KANG S J,LI Q,HU Y F.Deep desulfurization of gasoline using ion-exchange zeolites:Cu (I)-and Ag (I)-beta[J].Fuel Process Technol,2009,90:122-129. doi: 10.1016/j.fuproc.2008.08.003 [25] YI D Z,HUANG H,MENG X,SHI L.Adsorption-desorption behavior and mechanism of dimethyl disulfide in liquid hydrocarbon streams on modified Y zeolites[J].Appl Catal B:Environ,2014,148-149:377-386. doi: 10.1016/j.apcatb.2013.11.027 [26] SIEVERS C,LIEBERT J S,STRATMANN M M,OLINDO R,LERCHER J A.Comparison of zeolites LaX and LaY as catalysts for isobutane/2-butene alkylation[J].Appl Catal A:Gen,2008,336(1/2):89-100. https://www.researchgate.net/publication/244108235_Comparison_of_zeolites_LaX_and_LaY_as_catalysts_for_isobutane2-butene_alkylation [27] EMEIS C A.Determination of integrated molar extinction coefficients for infrared absorption bands of pyridine adsorbed on solid acid catalysts[J].J Catal,1993,141:347-354. doi: 10.1006/jcat.1993.1145 [28] EVERETT D H,POWL J C.Adsorption in slit-like and cylindrical micropores in the Henry's Law region[J].J Chem Soc Pakistan,1976,72:619-636. https://www.researchgate.net/publication/250836706_Adsorption_in_Slit-Like_Cylindrical_Micropores_in_the_Henry's_Law_Region [29] CHEN S G,YANG R T.Theoretical investigation of relationships between characteristic energy and pore size for adsorption in micropores[J].J Colloid Interf Sci,1996,177(2):298-306. doi: 10.1006/jcis.1996.0035 [30] 宋丽娟,潘明雪,秦玉才,鞠秀芳,段林海,陈晓陆.NiY分子筛选择性吸附脱硫性能及作用机理[J].高等学校化学学报,2011,30(3):787-792. http://www.cnki.com.cn/Article/CJFDTOTAL-GDXH201103061.htmSONG Li-juan,PAN Ming-xue,QIN Yu-cai,JU Xiu-fang,DUAN Lin-hai,CHEN Xiao-lu.Selective adsorption desulfurization performance and adsorptive mechanisms of NiY zeolites[J].Chem J Chin Univ,2011,30(3):787-792. http://www.cnki.com.cn/Article/CJFDTOTAL-GDXH201103061.htm -





 下载:
下载: