Influence of coal ash on potassium retention and ash fusibility during gasification of corn stalk coke
-
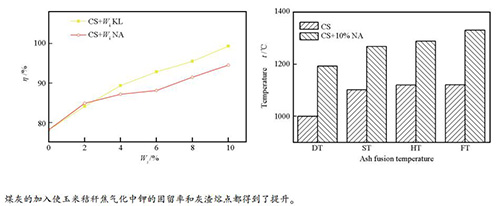
摘要: 在管式炉中,研究了煤灰对玉米秸秆焦在不同气化条件下钾的固留率和灰渣熔融性的影响。采用电感耦合等离子体发射光谱(ICP-AES)、X射线衍射(XRD)以及灰熔点测定仪等检测表征手段对气化灰渣中的钾元素含量、矿物质组成以及其熔融点进行了分析。实验以高岭土为参考,研究发现,煤灰如同高岭土具有一定的固钾能力,且随着添加量的增加钾固留率呈现增加的趋势,同时灰渣熔点也得到了提升。灰渣产物的XRD组成分析表明,挥发至气相中的钾以及灰渣中以熔融态存在的钾盐与煤灰中的硅铝化合物反应生成了高熔点的KAlSi3O8、KAlSi2O6和KAlSiO4,从而达到固钾的效果并提升了灰渣的熔点。Abstract: The potassium fixation ability and ash fusibility in gasification of corn stalk coke blended with coal ash were studied in CO2 atmosphere using a tube reactor. The ash samples were analyzed by inductively coupled plasma atomic emission spectrometer (ICP-AES), X-ray diffraction (XRD) and ash-melting point measuring device. The results show that coal ash has a certain ability of fixing potassium in the biomass ash as the reference of kaolin and the potassium retention ratio (PRR) increases when adding more coal ash. On the other hand, ash fusion temperatures (AFTs) of the blended ash increase by adding the coal ash, compared with the biomass ash. XRD patterns show that the reaction between alumina/silica compounds in coal ash and potassium that volatilized into the gas phase and existed in slag phase leads to formation of potassium aluminosilicates(KAlSi3O8, KAlSi2O6 and KAlSiO4), which are high melting point compounds. It confirms that coal ash is a potential additive for not only fixing potassium, but also increasing the ash fusion temperatures of easy-to-slagging biomass.
-
Key words:
- biomass /
- gasification /
- potassium retention /
- additive /
- ash fusibility
-
表 1 玉米秸秆的工业分析和元素分析
Table 1 Ultimate proximate and potassium analyses of the samples
Proximate analysis wad/% Ultimate analysis wad/% Element analysis wad/% M A V FC C H O* N St Kt 8.35 10.57 65.74 15.34 40.99 4.72 35.25 0.93 0.12 2.48 *: by difference; St: total sulfur; Kt: total potassium 表 2 玉米秸秆焦、宁夏煤和高岭土的灰化学成分分析
Table 2 Chemical compositions of ash of CS, NA and KL
Sample Chemical compositions w /% SiO2 Al2O3 Fe2O3 CaO MgO TiO2 SO3 K2O Na2O P2O5 CS 54.51 5.66 2.78 9.16 5.73 0.35 0.34 18.31 1.63 2.53 NA 50.11 38.33 6.30 0.61 0.42 0.84 0.36 0.58 0.16 0.07 KL 53.05 39.73 1.56 0.12 0.56 0.12 0.29 0.89 0.22 0.46 -
[1] SEO D K, LEE S K, KANG M W, HWANG J, YU T. Gasification reactivity of biomass chars with CO2[J]. Biomass Bioenergy, 2010, 34(12):1946-1953. doi: 10.1016/j.biombioe.2010.08.008 [2] 王燕杰, 应浩, 江俊飞.生物质二氧化碳气化综述[J].林产化学与工业, 2013, 33(6):121-127. http://d.old.wanfangdata.com.cn/Periodical/lchxygy201306023WANG Yan-jie, YING Hao, JIANG Jun-fei. A review of biomass carbon dioxide gasification[J]. Chem Ind Fore Prod, 2013, 33(6):121-127. http://d.old.wanfangdata.com.cn/Periodical/lchxygy201306023 [3] 徐婧. 生物质燃烧过程中碱金属析出的实验研究[D]. 杭州: 浙江大学, 2006.XU Jing. Experimental research on alkali release from biomass combustion[D]. Hangzhou: Zhejiang University, 2006. [4] 李九如, 李想, 陈巨辉, 孙佳伟.生物质气化技术进展[J].哈尔滨理工大学学报, 2017, 22(3):137-140. http://d.old.wanfangdata.com.cn/Periodical/heblgdxxb201703025LI Jiu-ru, LI Xiang, CHEN Ju-hui, SUN Jia-wei. Progress on technology of biomass gasification[J]. J Harbin Univ Sci Technol, 2017, 22(3):137-140. http://d.old.wanfangdata.com.cn/Periodical/heblgdxxb201703025 [5] HIROHATA O, WAKABAYASHI T, TASAKA K, FUSHIMI C, FURUSAWA T, KUCHONTHARA P, TSUTSUMI A. Release behavior of tar and alkali and alkaline earth metals during biomass steam gasification[J]. Energy Fuels, 2008, 22(6):4235-4239. doi: 10.1021/ef800390n [6] CHEN C, LUO Z, YU C, WANG T, ZHANG H. Transformation behavior of potassium during pyrolysis of biomass[J]. RSC Adv, 2017, 7(50):31319-31326. doi: 10.1039/C7RA05162J [7] 王茜, 韩奎华, 李辉, 齐建荟, 路春美. O2/CO2气氛下稻秆添加磷酸二氢铵对固钾及灰熔融特性的研究[J].燃料化学学报, 2015, 43(8):955-960. doi: 10.3969/j.issn.0253-2409.2015.08.008WANG Qian, HAN Kui-hua, LI Hui, QI Jian-hui, LU Chun-mei. Influence of ammonium dihydrogen phosphates additive on potassium fixation capacity and ash fusibility for rice straw combustion in an O2/CO2 atmosphere[J]. J Fuel Chem Technol, 2015, 43(8):955-960. doi: 10.3969/j.issn.0253-2409.2015.08.008 [8] GUO S, JIANG Y, LIU T, ZHAO J, HUANG J, FANG Y. Investigations on interactions between sodium species and coal char by thermogravimetric analysis[J]. Fuel, 2018, 214:561-568. doi: 10.1016/j.fuel.2017.11.069 [9] GUO S, JIANG Y, LIU T, ZHAO J, HUANG J, FANG Y. Optimization of leaching conditions for removing sodium from sodium-rich coals by orthogonal experiments[J]. Fuel, 2017, 208:499-507. doi: 10.1016/j.fuel.2017.07.032 [10] JENSEN P A, FRANDSEN F J, DAM-JOHANSEN K, SANDER B. Experimental investigation of the transformation and release to gas phase of potassium and chlorine during straw pyrolysis[J]. Energy Fuels, 2000, 14(6):1280-1285. doi: 10.1021/ef000104v [11] JOHANSEN J M, JAKOBSEN J G, FRANDSEN F J, GLARBORG P. Release of K, Cl, and S during pyrolysis and combustion of high-chlorine biomass[J]. Energy Fuels, 2011. 25(11):4961-4971. doi: 10.1021/ef201098n [12] 李琳娜, 任强强, 李诗媛, 吕清刚.富磷添加剂对麦秆燃烧过程中碱金属迁移转化行为的影响[J].中国电机工程学报, 2013, 33(26):41-47. http://d.old.wanfangdata.com.cn/Periodical/zgdjgcxb201326023LI Lin-na, REN Qiang-qiang, LI Shi-yuan, LÜ Qing-gang. Behavior of alkali metals during combustion of wheat straw with phosphorus-rich additives[J]. Proc CSEE, 2013, 33(26):41-47. http://d.old.wanfangdata.com.cn/Periodical/zgdjgcxb201326023 [13] TURN S Q, KINOSHITA C M, ISHIMURA D M, ZHOU J, HIRAKI T T, MASUTANI S M. A review of sorbent materials for fixed bed alkali getter systems in biomass gasifier combined cycle power generation applications[J]. J Energy Inst, 1998, 71(489):163-177. [14] BISWAS P, WU C Y. Control of toxic metal emissions from combustors using sorbents:A review[J]. J Air Waste Manage, 1998, 48(2):113-127. doi: 10.1080/10473289.1998.10463657 [15] LI Y, LI J, JIN Y, YOUQING W, GAO J. Study on alkali-metal vapor removal for high-temperature cleaning of coal gas[J]. Energy Fuels, 2005, 19(4):1606-1610. doi: 10.1021/ef049847x [16] LIU Y, DUAN X, CAO X, CHE D, LIU K. Experimental study on adsorption of potassium vapor in flue gas by coal ash[J]. Powder Technol, 2017, 318:170-176. doi: 10.1016/j.powtec.2017.05.024 [17] 任俊斌, 李俊国, 张永奇, 王志青, 李风海, 房倚天.生物质与烟煤混合灰熔融特性影响因素的研究[J].燃料化学学报, 2017, 45(11):1317-1322. doi: 10.3969/j.issn.0253-2409.2017.11.006REN Jun-bin, LI Jun-guo, ZHANG Yong-qi, WANG Zhi-qing, LI Feng-hai, FANG Yi-tian. Influence factors for fusion characteristics of mixed ash between biomass and bituminous coal[J]. J Fuel Chem Technol, 2017, 45(11):1317-1322. doi: 10.3969/j.issn.0253-2409.2017.11.006 [18] 郭帅, 蒋云峰, 熊青安, 宋双双, 赵建涛, 房倚天.准东煤热解过程中不同赋存形态钠变迁规律的研究[J].燃料化学学报, 2017, 45(3):257-264. doi: 10.3969/j.issn.0253-2409.2017.03.001GUO Shuai, JIANG Yun-feng, XIONG Qing-an, SONG Shuang-shuang, ZHAO Jian-tao, FANG Yi-tian. Release and transformation behaviors of sodium species with different occurrence modes during pyrolysis of Zhundong coal[J]. J Fuel Chem Technol, 2017, 45(3):257-264. doi: 10.3969/j.issn.0253-2409.2017.03.001 [19] 郭帅, 霍晓东, 宋双双, 蒋云峰, 赵建涛, 房倚天.高钠煤中钠的赋存形态研究[J].燃料化学学报, 2017, 45(10):1172-1177. doi: 10.3969/j.issn.0253-2409.2017.10.003GUO Shuai, HUO Xiao-dong, SONG Shuang-shuang, JIANG Yun-feng, ZHAO Jian-tao, FANG Yi-tian. Occurence modes of sodium species in sodium-rich coals[J]. J Fuel Chem Technol, 2017, 45(10):1172-1177. doi: 10.3969/j.issn.0253-2409.2017.10.003 [20] DAVIDSSON K O, MAND L, LECKNER B, KOVACEVIK B, SVANE M, HAGSTR M M, PETTERSSON J C, PETTERSSON J, ASTEMAN H. Potassium, chlorine, and sulfur in ash, particles, deposits, and corrosion during wood combustion in a circulating fluidized-bed boiler[J]. Energy Fuels, 2007, 21(1):71-81. doi: 10.1021/ef060306c [21] STEENARI B M, LINDQVIST O. High-temperature reactions of straw ash and the anti-sintering additives kaolin and dolomite[J]. Biomass Bioenergy, 1998, 14(1):67-76. doi: 10.1016/S0961-9534(97)00035-4 [22] CHEN Q, ZHOU J S, MEI Q F, LUO Z Y. The release behavior of potassium and sodium in the biomass high-temperature entrained-flow gasification[J]. Appl Mech Mater, 2011, 71/78:2434-2441. doi: 10.4028/www.scientific.net/AMM.71-78 [23] TRAN K Q, LISA K, STEENARI B M, LINDQVIST O. A kinetic study of gaseous alkali capture by kaolin in the fixed bed reactor equipped with an alkali detector[J]. Fuel, 2005, 84(2/3):169-175. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=JJ027711080 [24] KASSMAN H, PETTERSSON J, STEENARI B, AMAND L. Two strategies to reduce gaseous KCl and chlorine in deposits during biomass combustion-injection of ammonium sulphate and co-combustion with peat[J]. Fuel Process Technol, 2013, 105:170-180. doi: 10.1016/j.fuproc.2011.06.025 [25] 马孝琴, 秦建光, 骆仲泱, 余春江, 方梦祥, 岑可法.添加剂对稻草灰熔融特性影响的实验研究[J].浙江大学学报(工学版), 2010, 44(8):1573-1578. doi: 10.3785/j.issn.1008-973X.2010.08.025MA Xiao-qin, QIN Jian-guang, LUO Zhong-yang, YU Chun-jiang, FANG Meng-xiang, CEN Ke-fa. Effect of additives on fusion characteristic of ashes during rice straw combustion[J]. J Henan Agric Univ, 2010, 44(8):1573-1578. doi: 10.3785/j.issn.1008-973X.2010.08.025 [26] KEOWN D M, FAVAS G, HAYASHI J, LI C Z. Volatilisation of alkali and alkaline earth metallic species during the pyrolysis of biomass:differences between sugar cane bagasse and cane trash[J]. Bioresour Technol, 2005, 96(14):1570-1577. doi: 10.1016/j.biortech.2004.12.014 -





 下载:
下载: