Preparation of mesoporous Co-MCM-41 and its performance in adsorption removal of various basic nitrogen compounds
-
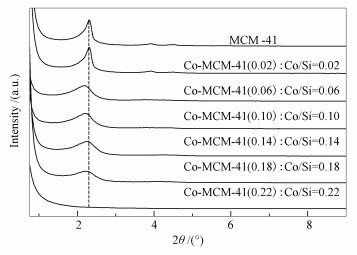
摘要: 采用水热法合成了MCM-41和不同Co/Si物质的量比的Co-MCM-41介孔材料, 并采用XRD、FT-IR和低温氮气吸附-脱附方法对样品进行了表征。FT-IR及XRD表征结果说明, Co原子已经进入了介孔材料的孔壁。合成的MCM-41及Co/Si(物质的量比)为0.18以下的Co-MCM-41都具有六方有序排列的介孔结构。当加入的Co/Si(物质的量比)为0.22时, 样品的(100)峰完全消失, 不具备六方有序排列的介孔结构, 说明以硝酸钴为钴源合成Co-MCM-41的最大Co加入量为Co/Si(物质的量比)为0.18左右。与MCM-41相比, 各Co-MCM-41样品的XRD(100)峰随着Co加入量的增加逐渐变宽变弱, 比表面积和孔容变小, 平均孔径增大。当加入的Co/Si物质的量比大于0.06时, Co-MCM-41的介孔孔道中存在少量聚集态的Co3O4。利用合成的Co-MCM-41吸附脱除氮含量为1737.35 μg/g模拟燃料中的碱性氮化物喹啉、苯胺或吡啶, 结果表明, 所有样品的吸附脱氮效果顺序为苯胺>吡啶>喹啉。Co-MCM-41(0.06)的吸附容量和氮脱除率明显要高于其他样品, 对苯胺、吡啶和喹啉的吸附容量分别为42.17、35.66和29.18 mg(N)/g, 去除率分别为82.38%、73.53%和61.11%。添加到模拟燃料中的芳烃化合物萘、苯或甲苯对其吸附脱氮没有影响, 表明介孔材料Co-MCM-41对各种含氮化合物的吸附主要是N原子与Co的配位络合吸附, 而不是π-π络合作用。采用焙烧或乙醇溶剂洗涤再生后的Co-MCM-41(0.06)恢复了吸附脱氮能力, 说明其具有较好的再生性能。Abstract: The mesoporous materials MCM-41 and Co-MCM-41, with Co/Si(molar ratio)=0.18, were prepared by hydrothermal synthesis method with cobalt nitrate as cobalt source and characterized by X-ray diffraction (XRD), fourier transform infrared spectrometry (FT-IR) and nitrogen adsorption-desorption.XRD and FT-IR results indicated that Co was introduced into the framework of mesoporous materials.The MCM-41 and Co-MCM-41 with highly ordered hexagonal mesoporous structure had been synthesized when the Co/Si(molar ratio) was 0.18 or less.But the sample had lost its ordered hexagonal mesoporous structure when Co/Si(molar ratio) was 0.22, indicating that the maximum addition amount of Co was about Co/Si(molar ratio)=0.18 when Co-MCM-41 was synthesized by using cobalt nitrate as cobalt source.Compared with the MCM-41, the intensity of XRD peak (100) of Co-MCM-41 became weak, broad and its surface area and total pore volume decreased, but the average pore diameter increased with the increase of Co amount.However, there was small amount of highly dispersed Co3O4 on the channel surface of Co-MCM-41 samples when the Co/Si(molar ratio) was 0.06 or more.Denitrification of model fuels containing about 1737.35 μg(nitrogen)/g of quinoline, aniline or pyridine was studied over the synthesized Co-MCM-41 with static adsorption at ambient conditions.The sequence of adsorption denitrification performance over all Co-MCM-41 samples was aniline, pyridine and quinoline.The adsorption capacity of Co-MCM-41(0.06) for aniline, pyridine and quinoline was 42.17, 35.66 and 29.18 mg(N)/g and the removal rate of basic nitrogen was 82.38%, 73.53% and 61.11% respectively.The coexisting aromatic compounds in model fuel had little impact on the removal performance of basic nitrogen over Co-MCM-41(0.06), implying that the N-M bond between the adsorption sites and N atom in the compound plays a significant role.Furthermore, Co-MCM-41 could be easily regenerated its adsorption denitrification performance by using calcination or ethanol regeneration method.
-
Key words:
- mesoporous materials /
- Co-MCM-41 /
- preparation /
- chatacterization /
- adsorption denitrification
-
表 1 MCM-41和Co-MCM-41的孔容、比表面积及平均孔径
Table 1 Total pore volumes, surface areas and average pore diameter of MCM-41 and Co-MCM-41
MCM-41 Co-MCM-41
(0.02)Co-MCM-41
(0.06)Co-MCM-41
(0.1)Co-MCM-41
(0.14)Co-MCM-41
(0.18)2θ/(°)(100) 2.3082 2.3065 2.1804 2.2203 2.2686 2.2321 d100/nm 3.8230 3.8257 4.0470 3.9743 3.8897 3.9533 a0/nm 4.4145 4.4177 4.6732 4.5892 4.4915 4.5650 Total pore volume v/(cm3·g-1) 0.8875 0.7756 0.7872 0.7452 0.8267 0.8021 ABET/(m2·g-1) 983 802 868 812 831 796 Average pore diameter d/nm 3.11 3.18 3.22 3.16 3.29 3.37 note:2d100sinθ=nλ; ${a_0} = \frac{{2{d_{100}}}}{{\sqrt 3 }}$ -
[1] 朱丽宁. 一季度全国机动车保有量突破3亿[N]. 人民公安报·交通安全周刊, 2017年4月18日, 第001版.ZHU Li-ning. The number of national vehicle has exceed 300 million[N]. China Police Daily Traffic Safety Weekly, 2017-4-18(001). [2] 肃宁, 杨一帆.我国成品油消费增速放缓[J].中国石油企业, 2017, (3):70-72. http://d.old.wanfangdata.com.cn/Periodical/zgsyqy201703014SU Ning, YANG Yi-fan.Oil products' consumption in China will mean slower growth remain slow[J].China Pet Enterprise, 2017, (3):70-72. http://d.old.wanfangdata.com.cn/Periodical/zgsyqy201703014 [3] 范武波, 陈军辉, 汪汀, 刘思宇, 钱骏, 叶宏.机动车尾气危害及对策研究[J].四川环境, 2015, 34(6):65-69. http://d.old.wanfangdata.com.cn/Periodical/schj201506013FAN Wu-bo, CHEN Jun-hui, WANG Ting, LIU Si-yu, QIAN Jun, YE Hong.Study on negative effects of vehicle emissions and countermeasures[J].Sichuan Environ, 2015, 34(6):65-69. http://d.old.wanfangdata.com.cn/Periodical/schj201506013 [4] 庞海全, 李艳芳, 韩冬云, 金阳, 乔海燕, 曹祖宾.烷基化法脱除模拟柴油中氮化物的研究[J].精细石油化工, 2017, 34(2):67-70. http://d.old.wanfangdata.com.cn/Periodical/jxsyhg201702016PANG Hai-quan, LI Yan-fang, HAN Dong-yun, JIN Yang, QIAO Hai-yan, CAO Zu-bin.Research on removing nitrogen compounds from model diesel oil by alkylation method[J].Spec Petrochem, 2017, 34(2):67-70. http://d.old.wanfangdata.com.cn/Periodical/jxsyhg201702016 [5] TAO X, ZHOU Y, WEI Q, DING S, ZHOU W.Inhibition effects of nitrogen compounds on deep hydrodesulfurization of straight-run gas oil over a NiW/Al2O3 catalyst[J].Fuel, 2017, 188:401-409. doi: 10.1016/j.fuel.2016.09.055 [6] HAN X, LIN H F, ZHENG Y.Adsorptive denitrogenation and desulfurization of diesel using activated carbons oxidized by (NH4)S2O8 under mild conditions[J].Can J Chem Eng, 2015, 93(3):538-548. doi: 10.1002/cjce.v93.3 [7] IMTEAZ A, SUNG H J.Remarkable improvement in adsorptive denitrogenation of model fossil fuels with CuCl/activated carbon, prepared under ambient condition[J].Chem Eng J, 2015, 279:327-334. doi: 10.1016/j.cej.2015.05.035 [8] BAE Y S, KIN M B, LEE H J, LEE C H, RYU J W.Adsorptive denitrogenation of light gas oil by silica-zirconia cogel[J].AIChE J, 2010, 52(2):510-521. doi: 10.1002/aic.10642/full [9] MUSHRUSH G W, QUINTANA M A, BAUSERMAN J W, WILLAUER H D.Post refining removal of organic nitrogen compounds from diesel fuels to improve environmental quality[J].J Environ Sci Health A, 2011, 46(2):176-180. doi: 10.1080/10934529.2011.532433 [10] SEO P W, AHMED I, JHUNG S H.Adsorptive removal of nitrogen containing compounds from a model fuel using a metal organic framework having a free carboxylic acid group[J].Chem Eng J, 2016, 299:236-243. doi: 10.1016/j.cej.2016.04.060 [11] 王朝阳, 李钢, 孙志国.磺酸功能化金属-有机骨架吸附脱氮性能[J].物理化学学报, 2013, 29(11):2422-2428. doi: 10.3866/PKU.WHXB201309021WANG Zhao-yang, LI Gang, SUN Zhi-guo.Denitrogenation through adsorption to sulfonated metal-organic frameworks[J].Acta Phys.-Chim Sin, 2013, 29(11):2422-2428. doi: 10.3866/PKU.WHXB201309021 [12] 洪新, 唐克.NaY分子筛的改性及吸附脱氮性能[J].燃料化学学报, 2015, 43(2):1-7. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18577.shtmlHONG Xin, TANG Ke.Modification and adsorptive denitrification of NaY molecular sieve[J].J Fuel Chem Technol, 2015, 43(2):1-7. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18577.shtml [13] HERNÁNDEZ M A J, YANG R T.Denitrogenation of transportation fuels by zeolites at ambient temperature and pressure[J].Angew Chem Int Ed, 2004, 43(8):1004-1006. doi: 10.1002/(ISSN)1521-3773 [14] 朱金柱, 沈健.SBA-15吸附脱除油品中的碱性氮化物[J].石油学报(石油加工), 2012, 40(11):566-570. http://d.old.wanfangdata.com.cn/Periodical/syxb-syjg201204007ZHU Jin-zhu, SHEN Jian.Adsorption of Basic Nitrogen Compounds from Oil by SBA-15 Zeolite[J].Acta Pet Sin (Pet Process Sect), 2012, 40(11):566-570. http://d.old.wanfangdata.com.cn/Periodical/syxb-syjg201204007 [15] SYED A S, LIN H F, ZHENG Y.Adsorptive denitrogenation and desulfurization of diesel fractions by mesoporous SBA15-supported nickel(Ⅱ) phosphide synthesized through a novel approach of urea matrix combustion[J].Ind Eng Chem Res, 2012, 51(44):14503-14510. doi: 10.1021/ie3015044 [16] ZHANG H, SONG H Y.Study of adsorptive denitrogenation of diesel fuel over mesoporous molecular sieves based on breakthrough curves[J].Ind Eng Chem Res, 2012, 51(50):16059-16065. https://www.researchgate.net/publication/263956669_Study_of_Adsorptive_Denitrogenation_of_Diesel_Fuel_over_Mesoporous_Molecular_Sieves_Based_on_Breakthrough_Curves [17] TANG K, HONG X.Preparation and characterization of Co-MCM-41 and its adsorption removing basic nitrogen compounds from FCC diesel oil[J].Energy Fuels, 2016, 30(6):4619-4624. doi: 10.1021/acs.energyfuels.6b00427 [18] 洪新, 唐克.杂原子介孔Co-MCM-41分子筛的制备及其吸附脱氮性能[J].燃料化学学报, 2015, 43(6):720-727. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18646.shtmlHONG Xin, TANG Ke.Preparation and adsorption denitrification of heteroatoms mesoporous molecular sieve Co-MCM-41[J].J Fuel Chem Technol, 2015, 43(6):720-727. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18646.shtml [19] 洪新, 唐克, 丁世洪.杂原子介孔Co-MCM-41分子筛的制备及其柴油深度吸附脱氮性能[J].燃料化学学报, 2016, 44(1):99-105. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18767.shtmlHONG Xin, TANG Ke.DING Shi-hong.Preparation and deep adsorption denitrification from diesel oil of heteroatoms mesoporous molecular sieve Co-MCM-41[J].J Fuel Chem Technol, 2016, 44(1):99-105. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract18767.shtml [20] KARTHIK M, TRIPATHI A K, GUPTA N M, VINU A, HARTMANN M, PALANICHAMY M, MURUGESAN V.Characteriaztion of Co, Al-MCM-41 and its activity in the t-butylation of phenol using isobutanol[J].Appl Catal A:Gen, 2004, 268(1):139-149. http://www.academia.edu/6609594/Characterization_of_Co_Al-MCM-41_and_its_activity_in_the_t-butylation_of_phenol_using_isobutanol [21] 孙庆林, 杨渊, 张颖杰, 李莎, 孙鹏, 孔岩.高钴含量MCM-41合成、催化性能及钴形态的化学分析方法[J].无机化学学报, 2011, 27(12):2346-2352. http://d.old.wanfangdata.com.cn/Periodical/wjhxxb201112004SUN Qing-lin, YANG Yuan, ZHANG Ying-jie, LI Sha, SUN Peng, KONG Yan.Synthesis, catalytic activity and chemical analysis method for Co-MCM-41 with high cobalt content[J].Chin J Inorg Chem, 2011, 27(12):2346-2352. http://d.old.wanfangdata.com.cn/Periodical/wjhxxb201112004 [22] PARVULESCU V, SU B L I.Cobalt or nickel substituted MCM-41 molecular sieves for oxidation of hydrocarbons[J].Catal Today, 2001, 69(1/4):315-322. https://www.researchgate.net/publication/223779525_Iron_cobalt_or_nickel_substituted_MCM-41_molecular_sieves_for_oxidation_of_hydrocarbons [23] LI Y N, GUO X H, ZHOU G D, HE X, BI Y L, LI W X, CHENG T X, WU T H, ZHEN K J.Estimation of consecutive and parallel reactions during ethane dehydrogenation with carbon dioxide over Co-MCM-41[J].Pol J Chem, 2005, 79(8):1357-1364. http://www.refdoc.fr/Detailnotice?cpsidt=17071008 [24] ANDRÉS A G B, MA G A, MA E R J, MARCELO N, M I O, KARIM S.Effect of the synthesis method on Co-catalysits based on MCM-41 for the fischer-tropsch reaction[J].Top Catal, 2011, 54(1/4):190-200. https://www.researchgate.net/profile/Karim_Sapag/publication/226873621_Effect_of_the_Synthesis_Method_on_Co-catalysts_Based_on_MCM-41_for_the_Fischer-Tropsch_Reaction/links/00b7d537e1f6c618ed000000/Effect-of-the-Synthesis-Method-on-Co-catalysts-Based-on-MCM-41-for-the-Fischer-Tropsch-Reaction.pdf [25] 李亚男, 郭晓红, 周广栋, 毕颖丽, 李文兴, 程铁欣, 吴通好, 甄开吉.Co-MCM-41催化剂上临CO2-乙烷脱氢反应的研究[J].高等学校化学学报, 2005, 26(6):1122-1125. http://www.whxb.pku.edu.cn/CN/abstract/abstract25734.shtmlLI Ya-nan, GUO Xiao-hong, ZHOU Guang-dong, BI Ying-li, LI Wen-xing, CHENG Tie-xin, WU Tong-hao, ZHEN Kai-ji.CO2-dehydrogenation of ethane over Co-MCM-41 catalyst[J].Chem J Chin Univ, 2005, 26(6):1122-1125. http://www.whxb.pku.edu.cn/CN/abstract/abstract25734.shtml [26] ÁGNES S, MARGARITA P, CHRISTO M.Catalytic activity of Co/MCM-41 and Co/SBA-15 materials in toluene oxidation[J].J Mater Sci, 2009, 44(24):6710-6716. doi: 10.1007/s10853-009-3600-y [27] 汤清虎, 赵培真, 张庆红, 王野.Co-MCM-41的表征及其催化苯乙烯环氧化性能[J].催化学报, 2005, 26(11):1031-1036. doi: 10.3321/j.issn:0253-9837.2005.11.021TANG Qing-hu, ZHAO Pei-zhen, ZHANG Qing-hong, WANG Ye.Characterization and catalytic performance of Co-MCM-41 for styrene epoxidation[J].Chin J Catal, 2005, 26(11):1031-1036. doi: 10.3321/j.issn:0253-9837.2005.11.021 [28] JIANG T S, SHEN W, ZHAO Q, LI M, CHU J Y, YIN H B.Characterization of CoMCM-41 mesoporous molecular sieves obtained by the microwave irradiation method[J].J Solid State Chem, 2008, 181(9):2298-2305. doi: 10.1016/j.jssc.2008.05.010 [29] 张燕, 李湘祁, 陈琼霞, 汤德平.微波法合成有序Co-MCM-41介孔分子筛[J].化工时刊, 2008, 22(2):12-15. http://d.old.wanfangdata.com.cn/Periodical/hgsk200802004ZHANG Yan, LI Xiang-qi, CHEN Qiong-xia, TANG De-ping.Microwave synthesis of ordered mesoporous Co-MCM-41[J].Chem Ind Time, 2008, 22(2):12-15. http://d.old.wanfangdata.com.cn/Periodical/hgsk200802004 [30] SHRIKANT S B, SINGH A P.Characterization and catalytic activity of cobalt containing MCM-41 prepared by direct hydrothermal, grafting and immobilization methods[J].J Mol Catal A:Chem, 2007, 266(1):118-130. https://www.sciencedirect.com/science/article/pii/S1381116906012349 [31] GALACHO C, CARROTT M M L R, CARROTT P J M.Structural and catalytic properties of Ti-MCM-41 synthesised at room temperature up to high Ti content[J].Microporous Mesoporous Mater, 2007, 100(1/3):312-321. https://www.sciencedirect.com/science/article/pii/S1387181106005154 [32] CHEN Y W, LIN H Y.Characteristics of Ti-MCM-41 and its catalytic properties in oxidation of benzene[J].J Porous Mat, 2002, 9(3):175-184. doi: 10.1023/A:1020982700613 [33] CHEN Y W, LU Y H.Characteristics of V-MCM-41 and its catalytic properties in oxidation of benzene[J].Ind Eng Chem Res, 1999, 38(5):1893-1903. doi: 10.1021/ie980665y [34] LEE D S, LIU T K.Characterization of V-MCM-41 mesoporous materials[J].J Sol-Gel Sci Technol, 2002, 24(1):69-80. doi: 10.1023/A:1015165600804 [35] 赵谦, 胡晓笑, 张蓉仙, 李梅, 姜廷顺.Co-MCM-41介孔分子筛的水热合成与稳定性[J].中国有色金属学报, 2009, 19(1):189-194. doi: 10.3321/j.issn:1004-0609.2009.01.030ZHAO Qian, HU Xiao-xiao, ZHANG Rong-xian, LI Mei, JIANG Ting-shun.Stability and hydrothermal synthesis of Co-MCM-41 mesoporous molecular sieves[J].Chin J Nonferrous Met, 2009, 19(1):189-194. doi: 10.3321/j.issn:1004-0609.2009.01.030 [36] ZHENG J, CHU W, ZHANG H, JIANG C F, DAI X Y.CO oxidation over Co3O4/SiO2 catalysts:Effects of porous structure of silica and catalyst calcination temperature[J].J Nat Gas Chem, 2010, 19(6):583-588. doi: 10.1016/S1003-9953(09)60119-5 [37] VARGHESE S, CUTRUFELLO M G, ROMBI E, CANNAS C, MONACI R, FERINO I.CO oxidation and preferential oxidation of CO in the presence of hydrogen over SBA-15-templated CuO-Co3O4 catalysts[J].Appl Catal A:Gen, 2012, 443/444(41):161-170. doi: 10.1081/CR-100104386?scroll=top&needAccess=true [38] 李亚男. 掺杂过渡金属的MCM-41介孔分分子筛的制备、表征及其对CO2乙烷氧化脱氢制乙烯的研究[D]. 长春: 吉林大学, 2006.LI Ya-nan. Synthesis, characterization and catalytic activity of Me-MCM-41 for oxidative dehydrogenation of ethane to ethylene with CO2[D]. Changchun: Jilin University, 2006. -





 下载:
下载: