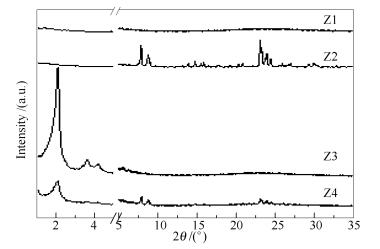
-
摘要: 多级孔结构ZSM-5分子筛的合成过程复杂。利用双模板剂, 通过优化晶化条件(如晶化时间与晶化温度) 和Si/Al物质的量比等一步水热晶化合成了具有多级孔结构的ZSM-5分子筛, 并采用XRD、N2吸附-脱附、吡啶红外吸脱附、SEM和TEM等方法对样品的晶体结构、孔道结构、表面酸性和形貌等进行了表征。结果表明, 一步法合成多级孔结构ZSM-5分子筛的适宜条件是:晶化温度160-180℃, 晶化时间24-96 h, 反应物组成为SiO2/Al2O3/Na2O/CTAB/TPABr/H2O=1/x/0.4/0.05/0.12/280, (x:50-240)。其中, 晶化温度160℃、晶化时间48 h和以Si/Al物质的量比50的凝胶合成的样品具有有序的介孔(平均尺寸3.60 nm) 结构、较高的结晶度和较强的酸性。Abstract: Generally, the process for synthesis of hierarchical-structured ZSM-5 zeolite is complex. Here, the polygonal three-dimensional ZSM-5 zeolite with hierarchical structure was hydrothermally synthesized by one-step synthesis method with dual templates. The effects of crystallization conditions and synthesis gel compositions, including crystallization temperature, crystallization time, Si/Al molar ratio, on the products were investigated for optimizing synthesis conditions. The X-ray diffraction (XRD), N2 adsorption-desorption experiment, Pyridine adsorption FT-IR (Py-FTIR), scanning electron microscopy (SEM) and transmission electron microscope (TEM) were used to characterize the crystalline structure, pore structure, surface acidity and crystal morphology of products. It was shown that the hierarchical-structured ZSM-5 zeolite can be synthesized under the following conditions: crystallization temperature of 160-180℃, crystallization time of 24-96 h, the SiO2/Al2O3/Na2O/CTAB/TPABr/H2O ratio of 1/x/0.4/0.05/0.12/280, (x: 50-240). The sample crystallized at 160℃ for 48 h with a synthesis gel having a Si/Al molar ratio of 50 had uniform cylindrical crystal morphology, high crystallinity and ordered mesoporous structure with a pore diameter of 3.60 nm. It contains both strong Brønsted and Lewis acid sites at 300℃, which contribute to its catalytic properties.
-
Key words:
- one-step synthesis method /
- dual templates /
- hierarchical structured /
- ZSM-5 /
- synthesis
-
Table 1 Conditions for synthesis of different ZSM-5 samples
Sample Crystallization temperature t/℃ Crystallization time t/h Molar ratio Si/Al CTAB/Si TPABr/Si Z1 160 24 50 0.00 0.00 Z2 160 24 50 0.00 0.15 Z3 160 24 50 0.12 0.00 Z4 160 24 50 0.12 0.15 Z5 140 24 50 0.12 0.15 Z6 180 24 50 0.12 0.15 Z7 160 24 25 0.12 0.15 Z8 160 24 120 0.12 0.15 Z9 160 48 50 0.12 0.15 Z10 160 72 50 0.12 0.15 Z11 160 108 50 0.12 0.15 Z12 160 48 25 0.12 0.15 Z13 160 48 40 0.12 0.15 Z14 160 48 80 0.12 0.15 Z15 160 96 50 0.12 0.15 Table 2 Surface areas and pore parameters of Z9 and Z13
Sample ABETa /(m2·g-1) Amicrob /(m2·g-1) v totalc /(cm3·g-1) v mesod /(cm3·g-1) Pore sizeed/nm micropore mesopore Z13 479.27 91.31 0.31 0.22 0.66 3.51 Z9 522.16 72.13 0.37 0.23 0.70 3.60 a: BET model; b: t-plot model; c: DFT model; d: BJH model; e: from adsorption branch of N2 adsorption isotherms -
[1] KOKOTAILO G T, LAWTON S L, OLSON D H, MEIER W M. Structure of synthetic zeolite ZSM-5[J]. Nature, 1978, 272:437-438. doi: 10.1038/272437a0 [2] CHOI M, NA K, KIM J, SAKAMOTO Y, TERASAKI O, RYOO R. Stable single-unit-cell nanosheets of zeolite MFI as active and long-lived catalysts[J]. Nature, 2009, 461(7261):246-249. doi: 10.1038/nature08288 [3] CORMA A, KUMAR D. Possibilities of mesoporous materials in catalysis[J]. Stud Surf Sci Catal, 1998, 117:201-222. doi: 10.1016/S0167-2991(98)80994-4 [4] BISCARDI J A, IGLESIA E. Structure and function of metal cations in light alkane reactions catalyzed by modified H-ZSM5[J]. Catal Today, 1996, 31(3/4):207-231. http://www.docin.com/p-1365951791.html [5] ZHU Z, CHEN Q, XIE Z, YANG W, KONG D, LI C. Shape-selective disproportionation of ethylbenzene to para-diethylbenzene over ZSM-5 modified by chemical liquid deposition and MgO[J]. J Mol Catal A:Chem, 2006, 248(1/2):152-158. https://www.researchgate.net/publication/244278254_Shape-selective_disproportionation_of_ethylbenzene_to_para-diethylbenzene_over_ZSM-5_modified_by_chemical_liquid_deposition_and_MgO [6] DEGNAN T F, CHITNIS G K, SCHIPPER P H. History of ZSM-5 fluid catalytic cracking additive development at Mobil[J]. Microporous Mesoporous Mater, 2000, 35-36:245-252. doi: 10.1016/S1387-1811(99)00225-5 [7] FIROOZI M, BAGHALHA M, ASADI M. The effect of micro and nano particle sizes of H-ZSM-5 on the selectivity of MTP reaction[J]. Catal Commun, 2009, 10(12):1582-1585. doi: 10.1016/j.catcom.2009.04.021 [8] ILIAS S, KHARE R, MALEK A, BHAN A. A descriptor for the relative propagation of the aromatic-and olefin-based cycles in methanol-to-hydrocarbons conversion on H-ZSM-5[J]. J Catal, 2013, 303:135-140. doi: 10.1016/j.jcat.2013.03.021 [9] FANG Y, TANG J, HUANG X, SHEN W, SONG Y, SUN C. Aromatization of dimethyl ether over Zn/H-ZSM-5 catalyst[J]. Chin J Catal, 2010, 31(3):264-266. doi: 10.1016/S1872-2067(09)60046-2 [10] RAO R R, SRINIVAS N, KULKARNI S J, SUBRAHMANYAM M, RAGHAVAN K V. A new route for the synthesis of 3, 5-lutidine over modified ZSM-5 catalysts[J]. Appl Catal A:Gen, 1997, 161(1/2):L37-L42. http://www.ingentaconnect.com/content/els/0926860x/1997/00000161/00000001/art00215 [11] CORMA A, NAVARRO M T. From micro to mesoporous molecular sieves:Adapting composition and structure for catalysis[J]. Stud Surf Sci Catal, 2002, 142:487-501. doi: 10.1016/S0167-2991(02)80065-9 [12] CORMA A. State of the art and future challenges of zeolites as catalysts[J]. J Catal, 2003, 216(1/2):298-312. [13] OGUNRONBI K E, AL-YASSIR N, AL-KHATTAF S. New insights into hierarchical metal-containing zeolites; synthesis and kinetic modelling of mesoporous gallium-containing ZSM-5 for propane aromatization[J]. J Mol Catal A:Chem, 2015, 406:1-18. doi: 10.1016/j.molcata.2015.05.005 [14] ROWNAGHI A A, REZAEI F, HEDLUND J. Uniform mesoporous ZSM-5 single crystals catalyst with high resistance to coke formation for methanol deoxygenation[J]. Microporous Mesoporous Mater, 2012, 151:26-33. doi: 10.1016/j.micromeso.2011.11.020 [15] CAI C, WANG H, HAN J. Synthesis and characterization of ionic liquid-functionalized alumino-silicate MCM-41 hybrid mesoporous materials[J]. Appl Surf Sci, 2011, 257(23):9802-9808. doi: 10.1016/j.apsusc.2011.06.025 [16] YASMIN T, MVLLER K. Structural characterization of alkyl bonded MCM-41 silica materials prepared by supercritical fluid approach[J]. Microporous Mesoporous Mater, 2015, 208:83-92. doi: 10.1016/j.micromeso.2015.01.035 [17] LEI J, FAN J, YU C, ZHANG L, JIANG S, TU B, ZHAO D. Immobilization of enzymes in mesoporous materials:Controlling the entrance to nanospace[J]. Microporous Mesoporous Mater, 2004, 73(3):121-128. doi: 10.1016/j.micromeso.2004.05.004 [18] KARLSSON A, STÖCKER M, SCHMIDT R. Composites of micro-and mesoporous materials:Simultaneous syntheses of MFI/MCM-41 like phases by a mixed template approach[J]. Microporous Mesoporous Mater, 1999, 27(2/3):181-192. [19] XIA Y, MOKAYA R. On the synthesis and characterization of ZSM-5/MCM-48 aluminosilicate composite materials[J]. J Mater Chem, 2004, 14(5):863-870. doi: 10.1039/b313389c [20] CHEN H, XI H, CAI X, QIAN Y. Experimental and molecular simulation studies of a ZSM-5-MCM-41 micro-mesoporous molecular sieve[J]. Microporous Mesoporous Mater, 2009, 118(1/3):396-402. [21] OGURA M, SHINOMIYA S Y, TATENO J, NARA Y, KIKUCHI E, MATSUKATA M. Formation of uniform mesopores in ZSM-5 zeolite through treatment in alkaline solution[J]. Chem Lett, 2000, 29(8):882-883. doi: 10.1246/cl.2000.882 [22] GROEN J C, PÉREZ-RAMÍREZ J, PEFFER L A. Formation of uniform mesopores in ZSM-5 zeolite upon alkaline post-treatment[J]. Chem Lett, 2002, 31(1):94-95. doi: 10.1246/cl.2002.94 [23] NA K, CHOI M, RYOO R. Recent advances in the synthesis of hierarchically nanoporous zeolites[J]. Microporous Mesoporous Mater, 2013, 166:3-19. doi: 10.1016/j.micromeso.2012.03.054 [24] JACOBSEN C J, MADSEN C, JANSSENS T V, JAKOBSEN H J, SKIBSTED J. Zeolites by confined space synthesis-characterization of the acid sites in nanosized ZSM-5 by ammonia desorption and27 Al/29Si-MAS NMR spectroscopy[J]. Microporous Mesoporous Mater, 2000, 39(1):393-401. https://www.researchgate.net/publication/223487379_Zeolites_by_Confined_Space_Synthesis-Characterisationof_the_Acid_Sites_in_Nanosized_ZSM-5_by_Ammonia_Desorption_and_27Al29Si-MAS_NMR_Spectroscopy [25] SCHMIDT I, BOISEN A, GUSTAVSSON E, STÅHL K, PEHRSON S, DAHL S, CARLSSON A, JACOBSEN C J. Carbon nanotube templated growth of mesoporous zeolite single crystals[J]. Chem Mater, 2001, 13(12):4416-4418. doi: 10.1021/cm011206h [26] JANSSEN A, SCHMIDT I, JACOBSEN C, KOSTER A, DE JONG K. Exploratory study of mesopore templating with carbon during zeolite synthesis[J]. Microporous Mesoporous Mater, 2003, 65(1):59-75. doi: 10.1016/j.micromeso.2003.07.003 [27] ZHAO J, HUA Z, LIU Z, LI Y, GUO L, BU W, CUI X, RUAN M, CHEN H, SHI J. Direct fabrication of mesoporous zeolite with a hollow capsular structure[J]. Chem Commun, 2009, (48):7578-7580. doi: 10.1039/b913920f [28] CHEN G, JIANG L, WANG L, ZHANG J. Synthesis of mesoporous ZSM-5 by one-pot method in the presence of polyethylene glycol[J]. Microporous Mesoporous Mater, 2010, 134(1/3):189-194. https://www.researchgate.net/publication/248293422_Synthesis_of_mesoporous_ZSM-5_by_one-pot_method_in_the_presence_of_polyethylene_glycol [29] ZHANG H, CHU L, XIAO Q, ZHU L, YANG C, MENG X, XIAO F. One-pot synthesis of Fe-Beta zeolite by an organotemplate-free and seed-directed route[J]. J Mater Chem A, 2013, 1(10):3254. doi: 10.1039/c3ta01238g [30] SING K, EVERETT D, HAUL R, MOSCOU L, PIEROTTI R, J. ROUQUÉ. SIEMIENIEWSKA T. Reporting physisorption data for gas/solid systems[R]. Pure Appl Chem, 1985, 57(4):603. [31] SING K S. Reporting physisorption data for gas/solid systems with special reference to the determination of surface area and porosity (Recommendations 1984)[R]. Pure Appl Chem, 1985, 57(4):603-619. -





 下载:
下载: