Effect of preparation method on the structure of rare earth metal Y modified Ni2P catalysts and its HDS performance
-
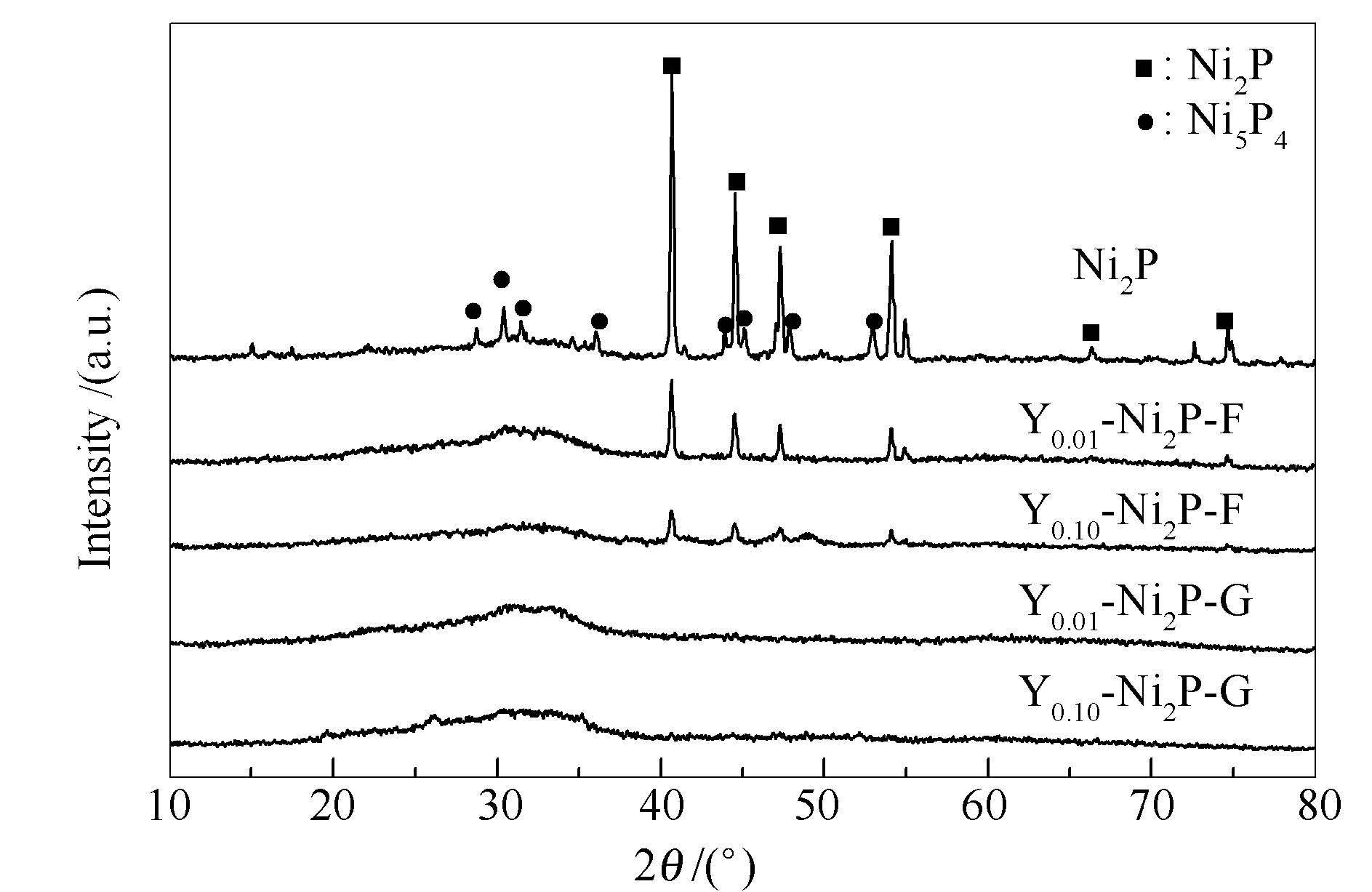
摘要: 采用一步法和分步法制备了钇(Y)改性的非负载型Yx-Ni2P催化剂(x为Y和Ni的物质的量比),并采用X射线衍射(XRD)、N2吸附比表面积(BET)测定、X射线光电子能谱(XPS)技术对催化剂的结构和性质进行了表征。以二苯并噻吩(DBT)为模型化合物,研究了制备方法对Yx-Ni2P催化剂加氢脱硫(HDS)性能的影响。结果表明,Y改性可以抑制Ni5P4杂晶相的生成,促进Ni2P活性相的生成,能显著提高催化剂的比表面积和孔容,从而有效提高磷化镍催化剂的HDS活性。Y/Ni物质的量比为0.10时,两种方法制备的催化剂均具有最高的HDS活性。与分步法相比,一步法制备得到的催化剂具有更大的比表面积和孔容,更小的表面P/Ni物质的量比,更高的CO吸附容量,暴露出更多的Ni活性位点,从而具有更高的HDS活性。在340℃,3.0 MPa,H2/油体积比为700,质量空速(WHSV)1.5 h-1的条件下,一步法制得的Y0.10-Ni2P催化剂上DBT HDS转化率达到97.7%,与分步法制备的Y0.10-Ni2P催化剂相比(92.3%),HDS活性提高了5.4%。Abstract: The yttrium (Y) modified unsupported Yx-Ni2P catalysts were prepared by one step method and stepwise method (x refers to mol ratio of Y to Ni), respectively.The catalysts were characterized by X-ray diffraction (XRD), N2 adsorption specific surface area measurements (BET), CO uptake and X-ray photoelectron spectroscopy (XPS).The effects of preparation methods on thehydrodesulfurization (HDS) property of the catalysts were investigated by using dibenzothiophene (DBT) as the model compound.The results show that the addition of Y can suppress the formation of Ni5P4 phase and thus promote the formation of active Ni2P phase.The addition of Y can dramatically increase the surface area and pore volume, effectively improve the HDS activity of nickel phosphide catalyst.The Yx-Ni2P catalysts prepared by these two methods with Y/Ni mol ratio of 0.10 exhibited the highest HDS activity.As compared to the stepwise method, the one step method which obtained catalysts possessed a larger specific surface area with high pore volume, a lower surface P/Ni mol ratio, a larger CO uptake and more exposed active Ni sites as compared to stepwise method.As a result, it showed a higher HDS activity.At a temperature of 340℃, a pressure of 3.0 MPa, a H2/oil volume ratio of 700 and a weight hourly space velocity (WHSV) of 1.5 h-1, the conversion of DBT over Y0.10-Ni2P catalyst prepared by one step method reached 97.7%, which was an increase of 5.4% comparing with the Y0.10-Ni2P catalyst prepared by stepwise method (92.3%).
-
Key words:
- one step method /
- stepwise method /
- hydrodesulfurization /
- nickel phosphide /
- yttrium /
- dibenzothiophene
-
表 1 Ni2P、Yx-Ni2P-G和Yx-Ni2P-F催化剂的表面结构和CO吸附性能
Table 1 Textural properties and CO uptake of the Ni2P,Yx-Ni2P-G and Yx-Ni2P-F catalysts
Sample ABET/(m2·g-1) vp/(cm3·g-1) d/nm CO uptake /(μmol·g-1) Ni2P 10 0.069 27.7 171 Y0.01-Ni2P-F 11 0.074 27.7 240 Y0.10-Ni2P-F 14 0.089 24.9 314 Y0.01-Ni2P-G 12 0.072 26.7 280 Y0.10-Ni2P-G 25 0.122 19.8 332 表 2 Ni2P、Yx-Ni2P-G和Yx-Ni2P-F催化剂的结合能
Table 2 XPS spectral parameters of the Ni2P,Yx-Ni2P-G and Yx-Ni2P-F catalysts
Sample Binding energy E/eV P/Ni
(mol ratio)Ni 2p3/2 P 2p Niδ+ Ni2+ satellite P5+ Pδ- Ni2P 852.7 856.7 861.2 134.8 128.8 3.0 Y0.10-Ni2P-F 852.6 856.6 860.9 134.6 130.3 2.4 Y0.10-Ni2P-G 852.5 856.5 860.8 134.5 128.6 1.4 -
[1] 于祺, 宋华, 宋华林, 王建, 姜楠, 李锋, 陈彦广. 还原温度对低温还原法制备的Ni2P/Ti-MCM-41催化剂的加氢脱硫性能的影响[J]. 燃料化学学报, 2016, 44(8):970-976. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18882.shtml(YU Qi, SONG Hua, SONG Hua-lin, WANG Jian, JIANG Nan, LI Feng, CHEN Yan-guang. Effect of reduction temperature on the performance of Ni2P/Ti-MCM-41 catalyst in hydrodesulfurization[J]. J Fuel Chem Technol, 2016, 44(8):970-976.) http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18882.shtml [2] 宋华, 代敏, 宋华林. Ni2P加氢脱硫催化剂[J]. 化学进展, 2012, 24(5):43-47.(SONG Hua, DAI Min, SONG Hua-lin. Ni2P catalyst for hydrodesulfurization[J]. Prog Chem, 2012, 24(5):43-47.) [3] WANG X, CLARK P, OYAMA S T. Synthesis, characterization and hydrotreating activity of several iron group transition metal phosphides[J]. J Catal, 2002, 208(2):321-331. doi: 10.1006/jcat.2002.3604 [4] OYAMA S T. Novel catalysts for advanced hydroprocessing:Transition metal phosphides[J]. J Catal, 2003, 216(1/2):343-352. https://www.researchgate.net/publication/223031296_Novel_catalysts_for_advanced_hydroprocessing_Transition_metal_phosphides [5] KORANYI T I, VIT Z, NAGY J B. Support and pretreatment effects on the hydrotreating activity of SBA-15 and CMK-5 supported nickel phosphide catalysts[J]. Catal Today, 2008, 130(1):80-85. doi: 10.1016/j.cattod.2007.09.004 [6] SAWHILL S J, PHILLIPS D C, BUSSELL M E. Thiophene hydrodesulfurization over supported nickel phosphide catalysts[J]. J Catal, 2003, 215(2):208-219. doi: 10.1016/S0021-9517(03)00018-6 [7] ZHAO H Y, OYAMA S T, FREUND H J, WLODARCZYK R, SIERKA M. Nature of active sites in Ni2P hydrotreating catalysts as probed by iron substitution[J]. Appl Catal B:Environ, 2015, 164:204-216. doi: 10.1016/j.apcatb.2014.09.010 [8] KORANYI T I. Phosphorus promotion of Ni(Co)-containing Mo-free catalysts in thiophene hydrodesulfurization[J]. Appl Catal A:Gen, 2003, 239(1/2):253-267. [9] HAYAO I, AKIRA O, EITETSU H, SUSUMU T. Rare earth metals as hydrogenation catalysts of unsaturated hydrocarbons[J]. J Catal, 1985, 96:139-145. doi: 10.1016/0021-9517(85)90367-7 [10] MAZZOCCHIA C, GRONCHI P, KADDOURI A, TEMPESTI E, ZANDERIGHI L, KIENNEMANN A. Hydrogenation of CO over Rh/SiO2-CeO2 catalysts:Kinetic evidences[J]. J Mol Catal A:Chem, 2001, 165(1/2):219-230. http://www.ingentaconnect.com/content/els/13811169/2001/00000165/00000001/art00425 [11] 霍晓敏, 陆清洁, 宋巍, 石雷. 稀土化合物在催化反应中的助剂作用[J]. 化学通报 (网络版), 2006, 69(1):w51.(HUO Xiao-min, LU Qing-jie, SONG wei, SHI Lei. The promoter function of rare earth compounds in catalytic reactions[J]. Chem Online, 2006, 69(1):w51.) [12] PHONTHAMMACHAI N, RUMRUANGWONG M, GULARI E, JAMIESON A M, JITKARNKA S, WONGKASEMJIT S. Synthesis and rheological properties of mesoporous nanocrystalline CeO2 via sol-gel process[J]. Colloids Surf A, 2004, 247:61-68. doi: 10.1016/j.colsurfa.2004.08.030 [13] 魏妮, 曾鹏辉, 季生福, 赵鹏飞, 刘辉, 李成岳. 镧助剂对Ni2P/SBA-15催化剂结构以及加氢脱硫性能的影响[J].中国稀土学报, 2011, 29(3):310-315.(WEI Ni, ZENG Peng-hui, JI Sheng-fu, ZHAO Peng-fei, LIU Hui, LI Cheng-yue. Effect of lanthanum promoter on structure and hydrodesulfurization performance of La-Ni2P/SBA-15 catalysts[J]. J Rare Earths, 2011, 29(3):310-315.) [14] SUN Z C, XIANG L, WANG A J, YAO W, YONG Y C. The effect of CeO2 on the hydrodenitrogenation performance of bulk Ni2P[J]. Top Catal, 2012, 55(14):1010-1021. [15] 宋华, 张福勇, 徐晓伟, 宋华林, 李峰. 镨和铈对低温还原法制备的Ni2P加氢脱硫活性的影响[J].燃料化学学报, 2015, 43(9):1128-1133. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18699.shtml(SONG Hua, ZHANG Fu-yong, XU Xiao-wei, SONG Hua-lin, LI Feng. Effect of Pr and Ce on the activity of Ni2P catalyst prepared by low temperature reduction in hydrodesulphurization[J]. J Fuel Chem Technol, 2015, 43(9):1128-1133.) http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18699.shtml [16] SONG H, XU X W, SONG H L, JIANG N, ZHANG F Y. The effect of neodymium content on dibenzothiophene HDS performance over a bulk Ni2P catalyst[J]. Catal Commun, 2015, 63:52-55. https://www.researchgate.net/profile/Hua_Song4/publication/277895780_The_effect_of_neodymium_content_on_dibenzothiophene_HDS_performance_over_a_bulk_Ni2P_catalyst/links/557eab3d08aec87640dc84a1.pdf?inViewer=true&disableCoverPage=true&origin=publication_detail [17] LAYMAN K A, BUSSELL M E. Infrared spectroscopic investigation of CO adsorption on silica-supported nickel phosphide catalysts[J]. J Phys Chem B, 2004, 108(30):10930-10941. doi: 10.1021/jp037101e [18] OYAMA S T, WANG X, LEE Y K, BANDO K, REQUEJO F G. Effect of phosphorus content in nickel phosphide catalysts studied by XAFS and other techniques[J]. J Catal, 2002, 210(1):207-217. doi: 10.1006/jcat.2002.3681 [19] CHEN T, YANG B L, LI S S, WANG K L, JIANG X D, ZHANG Y, HE G W. Ni2P catalysts supported on titania-modified alumina for the hydrodesulfurization of dibenzothiophene[J]. Ind Eng Chem Res, 2011, 50:11043-11048. doi: 10.1021/ie201188v [20] ELICHE-QUESADA D, MERIDA-ROBLES J, MAIRELES-TORRES P,RODRIGUEZ-CASTELLON E, BUSCA G, FINOCCHIO E, JIMENEZ-LOPEZ A. Effects of preparation method and sulfur poisoning on the hydrogenation and ring opening of tetralin on NiW/zirconium-dopedmesoporous silica catalysts[J]. J Catal, 2003, 220(2):457-467. doi: 10.1016/S0021-9517(03)00271-9 [21] KUHN J N, LAKSHMINARAYANAN N, OZKAN U S. Effect of hydrogen sulfide on the catalytic activity of Ni-YSZ cermets[J]. J Mol Catal A:Chem, 2008, 282(1/2):9-21. https://www.researchgate.net/publication/229288579_Effect_of_Hydrogen_Sulfide_on_the_Catalytic_Activity_of_Ni-YSZ_Cermets?_sg=9eAzwRjcifyh-bhtVUCY0YSGNC1PJuM9Sj1osbCzB3XofkPS45Y0_8c1BuBHG2s7bD2j2qmDmkMJra9-mQgzWHZ0oQZr14is3_uXbFOk2b8 [22] 郭亚男, 曾鹏辉, 季生福, 魏妮, 刘辉, 李成岳. Mo助剂含量对Mo-Ni2P/SBA-15/堇青石整体式催化剂加氢脱硫性能的影响[J]. 催化学报, 2010, 31(3):329-334.(GUO Ya-nan, ZENG Peng-hui, JI Sheng-fu, WEI Ni, LIU Hui, LI Cheng-yue. Effect of Mo promoter content on performance of Mo-Ni2P/SBA-15/cordierite monolithic catalyst for hydrodesulfurization[J]. Chin J Catal, 2010, 31(3):329-334.) [23] SONG H, WANG J, WANG Z D, SONG H L, LI F, JIN Z S. Effect of titanium content on dibenzothiophene HDS performance over Ni2P/Ti-MCM-41 catalyst[J]. J Catal, 2014, 311:257-265. doi: 10.1016/j.jcat.2013.11.021 [24] CECILIA J A, INFANTES-MOLINA A, RODRIGUEZ-CASTELLON E, JIMENEZ-LOPEZ A. A novel method for preparing an active nickel phosphide catalyst for HDS of dibenzothiophene[J]. J Catal, 2009, 263(1):4-15. doi: 10.1016/j.jcat.2009.02.013 [25] OYAMA S T, LEE Y K. The active site of nickel phosphide catalysts for the hydrodesulfurization of 4,6-DMDBT[J]. J Catal, 2008, 258(2):393-400. doi: 10.1016/j.jcat.2008.06.023 -





 下载:
下载: