-
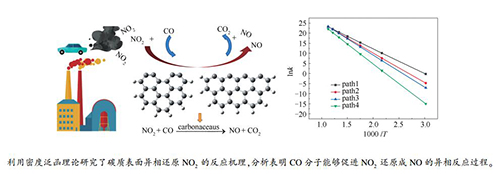
摘要: 基于量子化学密度泛函理论(DFT),研究了碳质表面异相还原NO2的反应机理,针对Zigzag与Armchair两种碳质表面,采用M06-2X方法与6-311G(d)基组联用,优化得到了不同反应路径下所有驻点的几何构型与能量,并对各路径进行了热力学与动力学分析,重点探究了CO在NO2异相还原反应中的作用规律,同时考察了碳质表面与反应温度对异相反应的影响。计算结果表明,NO2在碳质表面的异相还原过程主要分为两个阶段,即NO2还原阶段与碳氧化物释放阶段。通过对比无CO分子参与的反应可知,参与反应的CO分子可以降低各阶段的反应能垒并且加快各阶段的反应速率;CO分子存在时,NO2还原阶段的反应能垒被降低,促进了NO2还原成NO的异相反应过程,同时参与反应的CO分子与碳质表面剩余氧原子结合,形成CO2分子并释放,使碳氧化物释放阶段的反应能垒降低,从而促进了整体还原反应的进行。此外,与Armchair型相比,基于Zigzag型碳质表面的NO2异相还原反应能垒更低且反应速率更快,说明NO2异相还原反应更容易在Zigzag型碳质表面进行。最后,由反应动力学分析可知,随着温度上升,各阶段的反应速率均增大,说明提高温度对碳质表面的NO2异相还原能够起到促进作用。Abstract: Based on the quantum chemical density functional theory(DFT), the mechanism of heterogeneous reduction of NO2 on carbonaceous surface was studied. For zigzag and armchair carbonaceous surfaces, M06-2X method and 6-311G(d) basis set were used to optimize the geometry configuration and energy of all stagnation points under different reaction paths, and the reaction paths were analyzed and compared from thermodynamics and kinetics. The role of CO in the heterogeneous reduction of NO2 was deeply investigated, and the effects of carbon surface and reaction temperature on the heterogeneous reaction were also investigated. The results show that the heterogeneous reduction process of NO2 on the carbon surface can be divided into two stages: the reduction stage of NO2 and the desorption stage of carbon oxide. By comparing the reactions without CO molecules, it can be seen that the CO molecules involved in the reaction can reduce the reaction energy barrier of each stage and accelerate the reaction rate of each stage. In the presence of CO molecule, the reaction energy barrier at the reduction stage of NO2 is reduced, which promotes the heterogeneous reaction process of NO2 reduction to NO. CO molecules participating in the reaction can combine with the residual oxygen atoms on the surface to form and release CO2 molecules, which reduces the reaction energy barrier in the release stage of carbon oxides, thus promoting the overall reduction reaction. In addition, the energy barrier of NO2 heterogeneous reduction reaction on zigzag surface is lower and the reaction rate is faster than that on armchair surface, which indicates that the heterogeneous reduction reaction of NO2 is easier on Zigzag carbonaceous surface. Finally, the reaction kinetics analysis shows that the reaction rate of each stage increases with the increase of temperature, which indicates that increasing temperature can promote the heterogeneous reduction of NO2 on the carbonaceous surface.
-
Key words:
- NO2 /
- CO /
- carbonaceous surface /
- heterogeneous reduction /
- density functional theory /
- reaction kinetics
-
表 1 反应动力学参数
Table 1 Reaction kinetic parameters
Models Stages Pre-exponential
factor A / s-1Activation energy
Ea / (kJ·mol-1)Arrhenius equation Zigzag NO2 reduction with CO 2.90×1013 104.01 k=2.90×1013e-12508.41/T NO2 reduction without CO 1.40×1014 124.25 k=1.40×1014e-14943.92/T carbon oxide desorption with CO 9.49×1013 224.42 k=9.49×1013e-26991.54/T carbon oxide desorption without CO 5.44×1016 454.69 k=5.44×1016e-54686.77/T Armchair NO2 reduction with CO 3.54×1014 135.00 k=3.54×1014e-16236.87/T NO2 reduction without CO 9.03×1014 164.55 k=9.03×1014e-19790.39/T carbon oxide desorption with CO 3.94×1013 116.58 k=3.94×1013e-14021.60/T carbon oxide desorption without CO 3.58×1015 127.59 k=3.58×1015e-15345.05/T -
[1] 张秀霞, 吕晓雪, 伍慧喜, 谢苗, 林日亿, 周志军.钠对焦炭非均相还原NO的微观作用机理[J].燃料化学学报, 2020, 48(6):663-673. http://www.ccspublishing.org.cn/article/id/62451d3b-2053-47a0-b8f0-4234028f75aaZHANG Xiu-xia, LV Xiao-xue, WU Hui-xi, XIE Miao, LIN Ri-yi, ZHOU Zhi-jun. Microscopic mechanism for effect of sodium on NO heterogeneous reduction by char[J]. J Fuel Chem Technol, 2020, 48(6):663-673. http://www.ccspublishing.org.cn/article/id/62451d3b-2053-47a0-b8f0-4234028f75aa [2] 谈冠希, 迟姚玲, 李双, 易玉峰, 靳广洲.锰锆复合氧化物CO催化还原NO性能研究[J].燃料化学学报, 2019, 47(10):1258-1264. https://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CJFD&filename=RLHX201910013TAN Guan-xi, CHI Yao-ling, LI Shuang, YI Yu-feng, JIN Guang-zhou. Study on the performance of Mn-Zr composite oxide for CO reduction of NO[J]. J Fuel Chem Technol, 2019, 47(10):1258-1264. https://kns.cnki.net/KCMS/detail/detail.aspx?dbcode=CJFD&filename=RLHX201910013 [3] TAYLOR K. nitric oxide catalysis in automotive exhaust systems[J]. Catal Rev, 1993, 4(35):457-481. doi: 10.1080/01614949308013915 [4] WANG C, WANG P, DU Y, CHE D. Experimental study on effects of combustion atmosphere and coal char on NO2 reduction under oxy-fuel condition[J]. J Energy Ins, 2019, 92(4):1023-1033. doi: 10.1016/j.joei.2018.07.004 [5] 信晶.煤焦-NO反应过程中氮转化机理与试验研究[D].北京: 华北电力大学, 2015.XIN Jing. Mechanism and experimental study of nitrogen conversion in coal char-NO reaction process[D]. Beijing: North China Electric Power University, 2015. [6] 李竞岌, 杨欣华, 杨海瑞, 吕俊复.鼓泡床焦炭型氮氧化物生成的试验与模型研究[J].煤炭学报, 2016, 41(6):1546-1553. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=mtxb201606030LI Jing-ji, YANG Xin-hua, YANG Hai-rui, LV Jun-fu. Experimental and model study on the formation of coke type NOx in bubble bed[J].J China Coal Soc, 2016, 41(6):1546-1553. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=mtxb201606030 [7] WANG C A, DU Y, CHE D. Study on N2O reduction with synthetic coal char and high concentration CO during oxy-fuel combustion[J]. Proc Combust Inst, 2015, 35(2):2323-2330. doi: 10.1016/j.proci.2014.07.018 [8] 张秀霞, 周志军, 周俊虎, 姜树栋, 刘建忠, 岑可法. N2O在焦炭表面异相生成和分解机理的密度泛函理论研究[J].燃料化学学报, 2011, 39(11):806-811. http://d.wanfangdata.com.cn/periodical/rlhxxb201111002ZHANG Xiu-xia, ZHOU Zhi-jun, ZHOU Jun-hu, JIANG Shu-dong, LIU Jian-zhong, CEN Ke-fa. Density Functional Theory study on the mechanism of heterogeneous formation and decomposition of N2O on coke surface[J]. J Fuel Chem Technol, 2011, 39(11):806-811. http://d.wanfangdata.com.cn/periodical/rlhxxb201111002 [9] ARENILLAS A, ARIAS B, RUBIERA F, PIS J J, LÓPEZ R, CAMPOMANES P, PEVIDA C, MENÉNDEZ M I. Heterogeneous reaction mechanisms of the reduction of nitric oxide on carbon surfaces:A theoretical analysis[J]. Theor Chem Acc, 2010, 127(1/2):95-108. doi: 10.1007/s00214-009-0708-8 [10] PEVIDA C, ARENILLAS A, RUBIERA F, PIS J J. Synthetic coal chars for the elucidation of NO heterogeneous reduction mechanisms[J]. Fuel, 2007, 86(1/2):41-49. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=1202d52ac2263a66c1d375b9adda482a [11] 应芝, 郑晓园, 崔国民.基于O2/CO2气氛的煤粉燃烧性能研究[J].动力工程学报, 2019, 39(1):7-12. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=dlgc201901002YING Zhi, ZHENG Xiao-yuan, CUI Guo-min. Study on combustion performance of pulverized coal based on O2/CO2 atmosphere[J].Chin J Power Eng, 2019, 39(1):7-12. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=dlgc201901002 [12] DEGRAEUWE B, THUNIS P, CLAPPIER A, WEISS M, LEFEBVRE W, JANSSEN S, VRANCKX S. Impact of passenger car NOx emissions and NO2 fractions on urban NO2 pollution-Scenario analysis for the city of Antwerp, Belgium[J]. Atmos Environ, 2016, 126:218-224. doi: 10.1016/j.atmosenv.2015.11.042 [13] 欧阳子区, 朱建国, 吕清刚.无烟煤粉经循环流化床预热后燃烧特性及NOx排放特性实验研究[J].中国电机工程学报, 2014, (11):1748-1754. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgdjgcxb201411005OUYANG Zi-qu, ZHU Jian-guo, LV Qing-gang. Experimental study on combustion and NOx emission of pulverized anthracite coal preheated by a circulating fluidized bed[J]. Proc CSEE, 2014, (11):1748-1754. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgdjgcxb201411005 [14] GARCÍA-GARCÍA A, ILLÁN-GÓMEZ M J, LINARES-SOLANO A, SALINAS-MARTÍNEZ DE LECEA C. NOx Reduction by potassium-containing coal briquettes. effect of NO2 concentration[J]. Energy Fuels, 1999, 13(2):499-505. doi: 10.1021/ef980165h [15] SADAOKA Y, JONES T A, REVELL G S, G PEL W. Effects of morphology on NO2 detection in air at room temperature with phthalocyanine thin films[J]. J Mater Sci, 1990, 25(12):5257-5268. doi: 10.1007/BF00580159 [16] ZHU X, ZHANG L, ZHANG M, MA C. Effect of N-doping on NO2 adsorption and reduction over activated carbon:An experimental and computational study[J]. Fuel, 2019, 258:116109. doi: 10.1016/j.fuel.2019.116109 [17] JEGUIRIM M, TSCHAMBER V, BRILHAC J F, EHRBURGER P. Interaction mechanism of NO2 with carbon black:effect of surface oxygen complexes[J]. J Anal Appl Pyrolysis, 2004, 72(1):171-181. doi: 10.1016/j.jaap.2004.03.008 [18] MUCKENHUBER H, GROTHE H. The heterogeneous reaction between soot and NO2 at elevated temperature[J]. Carbon, 2006, 44(3):546-559. doi: 10.1016/j.carbon.2005.08.003 [19] 王春波, 岳爽, 许旭斌, 李一鹏. O2/CO2气氛下煤焦恒温燃烧NOx释放特性[J].煤炭学报, 2018, 43(1):257-264. http://www.cqvip.com/QK/96550X/20181/674694153.htmlWANG Chun-bo, YUE Shuang, XU Xu-bin, LI Yi-peng. NOx emission characteristics of coal coke under constant temperature combustion in O2/CO2 atmosphere[J]. J China Coal Soc, 2018, 43(1):257-264. http://www.cqvip.com/QK/96550X/20181/674694153.html [20] 王贲, 苏胜, 孙路石, 胡松, 费华, 卢腾飞, 向军. O2/CO2气氛下CO对煤焦异相还原NO影响的研究[J].工程热物理学报, 2012, 33(2):336-338. http://www.cnki.com.cn/Article/CJFDTotal-GCRB201202042.htmWANG Bi, SU Sheng, SUN Lu-shi, HU Song, FEI Hua, LU Teng-fei, XIANG Jun. Study on the effect of CO on NO reduction in coal coke under O2/CO2 atmosphere[J]. J Eng Thermo, 2012, 33(2):336-338. http://www.cnki.com.cn/Article/CJFDTotal-GCRB201202042.htm [21] 魏帅, 严国超, 张志强, 刘松孟, 张远方.晋城无烟煤的分子结构特征分析[J].煤炭学报, 2018, 43(2):555-562.WEI Shuai, YAN Guo-chao, ZHANG Zhi-qiang, LIU Meng-song, ZHANG Yuan-fang. Analysis of molecular structure characteristics of Jincheng Anthracite[J]. J China Coal Soc, 2018, 43(2):555-562. [22] 邹潺, 王春波, 邢佳颖.煤燃烧过程中砷与氮氧化物的反应机理[J].燃料化学学报, 2019, 47(2):138-143. http://www.ccspublishing.org.cn/article/id/c24a55cc-9570-4473-8d94-6a0c372968b1ZOU Chan, WANG Chun-bo, XING Jia-ying. Reaction mechanism of arsenic and nitrous oxides during coal combustion[J]. J Fuel Chem Technol, 2019, 47(2):138-143. http://www.ccspublishing.org.cn/article/id/c24a55cc-9570-4473-8d94-6a0c372968b1 [23] SINGLA P, SINGHAL S, GOEL N. Theoretical study on adsorption and dissociation of NO2 molecules on BNNT surface[J]. Appl Surf Sci, 2013, 283:881-887. doi: 10.1016/j.apsusc.2013.07.038 [24] SHENG C. Char structure characterised by Raman spectroscopy and its correlations with combustion reactivity[J]. Fuel, 2007, 86(15):2316-2324. doi: 10.1016/j.fuel.2007.01.029 [25] ENOKI T, FUJⅡ S, TAKAI K. Zigzag and armchair edges in graphene[J]. Carbon, 2012, 50(9):3141-3145. doi: 10.1016/j.carbon.2011.10.004 [26] GIRITÇÖ, MEYER J C, ERNI R, ROSSELL M D, KISIELOWSKI C, YANG L, PARK C, CROMMIE M F, COHEN M L, LOUIE S G, ZETTL A. Graphene at the edge:Stability and dynamics[J]. Science, 2009, 323(5922):1705-1708. doi: 10.1126/science.1166999 [27] JIAO A, ZHANG H, LIU J, JIANG X. Quantum chemical and kinetics calculations for the NO reduction with char(N):Influence of the carbon monoxide[J]. Combust Flame, 2018, 196:377-385. doi: 10.1016/j.combustflame.2018.06.029 [28] 余岳溪, 高正阳, 季鹏, 李方勇, 杨维结.煤焦异相还原N2O的反应机理[J].化工学报, 2017, 68(1):369-374. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hgxb201701044YU Yue-xi, GAO Zheng-yang, JI Peng, LI Fang-yong, YANG Wei-jie. Reaction mechanism of heterogeneous reduction of N2O from coal coke[J]. CIESC J, 2017, 68(1):369-374. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hgxb201701044 [29] CHEN N, YANG R T. Ab initio molecular orbital calculation on graphite:Selection of molecular system and model chemistry[J]. Carbon, 1998, 36(7):1061-1070. http://jnumedmtg.snmjournals.org/cgi/content/short/53/1_MeetingAbstracts/1540 [30] YANG F H, YANG R T. Ab initio molecular orbital study of adsorption of atomic hydrogen on graphite:Insight into hydrogen storage in carbon nanotubes[J]. Carbon, 2002, 40(3):437-444. doi: 10.1016/S0008-6223(01)00199-3 [31] GAO Z, YANG W, DING X, DING Y, YAN W. Theoretical research on heterogeneous reduction of N2O by char[J]. App Thermal Eng 2017, 126:28-36. doi: 10.1016/j.applthermaleng.2017.07.166 [32] JIAO A, ZHANG H, LIU J, SHEN J, JIANG X. The role of CO played in the nitric oxide heterogeneous reduction:A quantum chemistry study[J]. Energy, 2017, 141:1538-1546. doi: 10.1016/j.energy.2017.11.115 [33] ZHAO Y, TRUHLAR D G. The M06 suite of density functionals for main group thermochemistry, thermochemical kinetics, noncovalent interactions, excited states, and transition elements:Two new functionals and systematic testing of four M06 functionals and 12 other functionals[J]. Theor Chem Acc, 2008, 119(5/6):525. doi: 10.1007/s00214-007-0310-x [34] ZHAO Y, TRUHLAR D G. A Prototype for graphene material simulation:Structures and interaction potentials of coronene dimers[J]. J Phys Chem C, 2008, 112(11):4061-4067. doi: 10.1021/jp710918f [35] 高正阳, 杨维结, 阎维平.煤焦催化HCN还原NO的反应机理[J].燃料化学学报, 2017, 45(9):1043-1048. http://www.cnki.com.cn/Article/CJFDTotal-RLHX201709003.htmGAO Zheng-yang, YANG Wei-jie, YAN Wei-ping. Reaction mechanism of coal coke catalyzing HCN reduction[J]. J Fuel Chem Technol, 2017, 45(9):1043-1048. http://www.cnki.com.cn/Article/CJFDTotal-RLHX201709003.htm [36] J F M, W T G, B S H, E S G, A R M, et al. Revision A.03[CP]. Wallingford CT: Gaussian, Inc., 2016. [37] ZHANG H, LIU J, SHEN J, JIANG X. Thermodynamic and kinetic evaluation of the reaction between NO (nitric oxide) and char(N) (char bound nitrogen) in coal combustion[J]. Energy, 2015, 82:312-321. doi: 10.1016/j.energy.2015.01.040 [38] 许紫阳, 岳爽, 王春波, 刘瑞琪.焦炭催化CO还原NO的反应机理研究[J].燃料化学学报, 2020, 48(3):266-274. http://www.cnki.com.cn/Article/CJFDTotal-RLHX20200416006.htmXU Zi-yang, YUE Shuang, WANG Chun-bo, LIU Rui-qi. Study on the reaction mechanism of NO reduction with CO catalyzed by char[J]. J Fuel Chem Technol, 2020, 48(3):266-274. http://www.cnki.com.cn/Article/CJFDTotal-RLHX20200416006.htm [39] 钟俊, 高正阳, 丁艺, 余岳溪, 杨维结. Zigzag煤焦表面异相还原N2O反应[J].煤炭学报, 2017, 42(11):3028-3034. http://www.cnki.com.cn/Article/CJFDTotal-MTXB201711031.htmZHONG Jun, GAO Zheng-yang, DING Yi, YU Yue-xi, YANG Wei-jie. Heterogeneous reduction of N2O reaction on Zigzag coal char surface[J].J China Coal Soc, 2017, 42(11):3028-3034. http://www.cnki.com.cn/Article/CJFDTotal-MTXB201711031.htm [40] CHEN P, GU M, CHEN G, LIU F, LIN Y. DFT study on the reaction mechanism of N2O reduction with CO catalyzed by char[J]. Fuel, 2019, 254:115666. doi: 10.1016/j.fuel.2019.115666 [41] 王鹏乾, 王长安, 杜勇博, 张龙飞, 车得福. O2/CO2燃烧条件下NO2还原特性的实验研究[J].西安交通大学学报, 2017, 51(5):16-22. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=xajtdxxb201705003WANG Peng-qian, WANG Chang-an, DU Yong-bo, ZHANG Long-fei, CHE De-fu. Experimental study on NO2 reduction characteristics under O2/CO2 combustion conditions[J]. J Xi'an Jiaotong Univ, 2017, 51(5):16-22. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=xajtdxxb201705003 -





 下载:
下载: