Preparation of the Nb-P/SBA-15 catalyst and its performance in the dehydration of fructose to 5-hydroxymethylfurfural
-
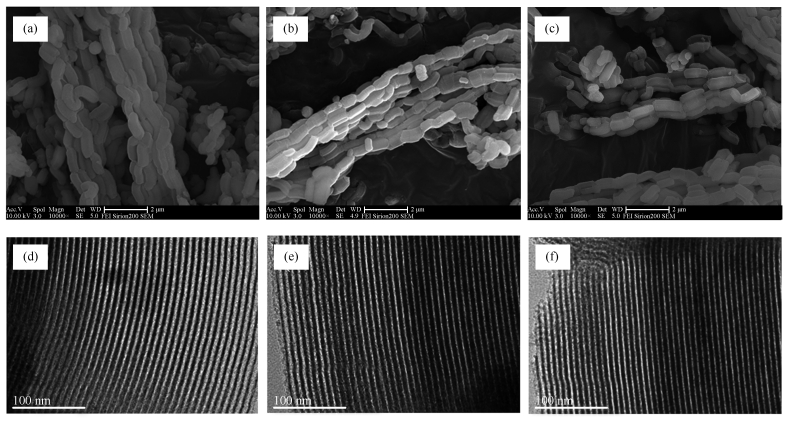
摘要: 果糖催化脱水制5-羟甲基糠醛(5-HMF)是生物质转化制高附加值化合物过程中的一个重要反应。采用自制的介孔SBA-15,浸渍法制备了Nb/SBA-15催化剂,再经磷酸化处理制得Nb-P/SBA-15催化剂,研究了该催化剂在果糖脱水制5-HMF反应中的性能。SEM、TEM、BET和XRD表征结果表明,Nb/SBA-15和Nb-P/SBA-15完好地保留了SBA-15的微观结构,其内孔道直径为10 nm,铌酸在孔内表面分布均匀;负载铌和磷酸化后,孔壁变薄。NH3-TPD结果显示,Nb/SBA-15经磷酸预处理后,不仅弱酸性位有所增加,而且产生了中强酸和强酸性位,使得在含水两相体系果糖脱水反应中,Nb-P/SBA-15比Nb/SBA-15具有更高的催化活性和5-HMF选择性。同时考察了反应温度、溶剂比例、反应时间对果糖脱水的影响,结果表明,以水/MIBK(V/V=1/2)为溶剂时,160 ℃下反应1.5 h,果糖转化率和5-HMF收率分别高达96.1%和92.6%。Nb-P/SBA-15经循环使用四次后仍具有较好的催化活性,表明该催化剂具有较高的耐水稳定性。Abstract: Dehydration of fructose to 5-hydroxymethylfurfural (5-HMF) is one of the pivotal reactions in conversion of biomass towards valuable platform compounds. In this work, Nb/SBA-15 was prepared via incipient wetness impregnation with self-made SBA-15 as support; Nb/SBA-15 was further treated with phosphoric acid and calcined at 450℃, to obtain the Nb-P/SBA-15 catalyst. The Nb-P/SBA-15 catalyst was characterized by SEM, TEM, BET, XRD and NH3-TPD; its performance in the dehydration of fructose to 5-HMF was then investigated. The results indicate that the microscopic structure of SBA-15 is well preserved in Nb/SBA-15 with an internal channel diameter of about 10 nm and the niobic species are highly dispersed on the surface of the channels; the wall of channels became thinner after impregnation of Nb and treatment with phosphoric acid. After the treatment with phosphoric acid, the weak acid sites are increased, moreover, the medium and strong acidic sites are generated in Nb-P/SBA-15; as a result, for the dehydration of fructose in a water/MIBK biphasic system, Nb-P/SBA-15 exhibits higher catalytic activity and selectivity to 5-HMF. By reaction at 160℃ for 1.5 h, with a water/MIBK volume ratio of 1/2, the conversion of fructose and the yield of 5-HMF reach 96.1% and 92.6%, respectively. Moreover, the Nb-P/SBA-15 catalyst also exhibits excellent stability in view of water tolerance; it still demonstrates high catalytic activity and selectivity to 5-HMF even after successive recycling for four times.
-
Key words:
- niobic acid /
- phosphoric treatment /
- fructose /
- hydration /
- 5-hydroxymethyl furfural (5-HMF) /
- Nb-P/SBA-15
-
Table 1 Textural properties and acidity of various catalysts

Table 2 Fructose dehydration to 5-HMF in different solvents

-
[1] 林木森, 蒋建春.生物质快速热解技术现状[J].生物质化学工程, 2006, 40(1):21-26. http://www.cnki.com.cn/Article/CJFDTOTAL-LCHG200601006.htmLIN Mu-sen, JIANG Jian-chun. A review on fast pyrolysis of biomass[J]. Biomass Chem Eng, 2006, 40(1):21-26. http://www.cnki.com.cn/Article/CJFDTOTAL-LCHG200601006.htm [2] RUSSO P A, ANTUNES M A, NEVES P, WIPER P V, FAZIO E, NERI F, BARRECA F, MAFRA L, PILLINGER M, PINNA N, VALENTE A A. Mesoporous carbon-silica solid acid catalysts for producing useful bio-products within the sugar-platform of biorefineries[J]. Green Chem, 2014, 16(9):4292-4305. doi: 10.1039/C4GC01037J [3] OSATIASHTIANI A, LEE A F, GRANOLLERS M, BROWN D R, OLIVI L, MORALES G, MELERO J A, WILSON K. Hydrothermally stable, conformal, sulfated zirconia monolayer catalysts for glucose conversion to 5-HMF[J]. ACS Catal, 2015, 5(7):4345-4352. doi: 10.1021/acscatal.5b00965 [4] CHOUDHARY V, MUSHRIF S H, HO C, ANDERKO A, NIKOLAKIS V, MARINKOVIC N S, FRENKEL A I, SANDLER S I, VLACHOS D G. Insights into the interplay of Lewis and Brønsted acid catalysts in glucose and fructose conversion to 5-(hydroxymethyl)furfural and levulinic acid in aqueous media[J]. J Amer Chem Soc, 2013, 135(10):3997-4006. doi: 10.1021/ja3122763 [5] TAKEUCHI Y, JIN F, TOHJI K, ENOMOTO H. Acid catalytic hydrothermal conversion of carbohydrate biomass into useful substances[J]. J Mate Sci, 2007, 43(7):2472-2475. doi: 10.1007/s10853-007-2021-z [6] SOUZA R L D, YU H, RATABOUL F, ESSAYEM N. 5-hydroxymethylfurfural (5-HMF) production from hexoses:Limits of heterogeneous catalysis in hydrothermal conditions and potential of concentrated aqueous organic acids as reactive solvent system[J]. Challenges, 2012, 3(2):212-232. doi: 10.3390/challe3020212 [7] MORENO-RECIO M, SANTAMARÍA-GONZÁLEZ J, MAIRELES-TORRES P. Brønsted and Lewis acid ZSM-5 zeolites for the catalytic dehydration of glucose into 5-hydroxymethylfurfural[J]. Chem Eng J, 2016, 303:22-30. doi: 10.1016/j.cej.2016.05.120 [8] SARAVANAMURUGAN S, PANIAGUA M, MELERO JA, RⅡSAGER A. Efficient isomerization of glucose to fructose over zeolites in consecutive reactions in alcohol and aqueous media[J]. J Am Chem Soc, 2013, 135(14):5246-5249. doi: 10.1021/ja400097f [9] CUI M, HUANG R, QI W, SU R, HE Z. Cascade catalysis via dehydration and oxidation:One-pot synthesis of 2, 5-diformylfuran from fructose using acid and V2O5/ceramic catalysts[J]. RSC Adv, 2017, 7(13):7560-7566. doi: 10.1039/C6RA27678D [10] JIMÉNEZ-MORALES I, MORENO-RECIO M, SANTAMARÍA-GONZÁLEZ J, MAIRELES-TORRES P, JIMÉNEZ-LÓPEZ A. Mesoporous tantalum oxide as catalyst for dehydration of glucose to 5-hydroxymethylfurfural[J]. Appl Catal B:Environ, 2014, 154-155:190-196. doi: 10.1016/j.apcatb.2014.02.024 [11] XUE Z, MA MG, LI Z, MU T. Advances in conversion of glucose and cellulose to 5-hydroxymethylfurfural over heterogeneous catalysts[J]. RSC Adv, 2016, 6:98874-98892. doi: 10.1039/C6RA20547J [12] XIAO Y, SONG Y F. Efficient catalytic conversion of the fructose into 5-hydroxymethylfurfural by heteropolyacids in the ionic liquid of 1-butyl-3-methyl imidazolium chloride[J]. Appl Catal A:Gen, 2014, 484:74-78. doi: 10.1016/j.apcata.2014.07.014 [13] LI Y, LIU H, SONG C, GU X, LI H, ZHU W, YIN S, HAN C. The dehydration of fructose to 5-hydroxymethylfurfural efficiently catalyzed by acidic ion-exchange resin in ionic liquid[J]. Bioresour Technol, 2013, 133:347-353. doi: 10.1016/j.biortech.2013.01.038 [14] SAMPATH G, KANNAN S. Fructose dehydration to 5-hydroxymethylfurfural:Remarkable solvent influence on recyclability of Amberlyst-15 catalyst and regeneration studies[J]. Catal Commun, 2013, 37:41-44. doi: 10.1016/j.catcom.2013.03.021 [15] NAKAJIMA K, BABA Y, NOMA R, KITANO M, KONDO J N, HAYASHI S, HARA M. Nb2O5·nH2O as a heterogeneous catalyst with water-tolerant Lewis acid sites[J]. J Am Chem Soc, 2011, 133(12):4224-4227. doi: 10.1021/ja110482r [16] MAURER S M, KO E I. ChemInform abstract:Structural and acidic characterization of niobia aerogels[J]. ChemInform, 1992, 135(30):125-134. doi: 10.1002/chin.199230010/full [17] RAMANATHAN A, ZHU H, MAHESWARI R, THAPA P S, SUBRAMANIAM B. Comparative study of Nb-incorporated cubic mesoporous silicates as epoxidation catalysts[J]. Ind Eng Chem Res, 2015, 54(16):4236-4242. doi: 10.1021/ie504386g [18] YANG Z J, LI Y F, WU Q B, REN N, ZHANG Y H, LIU Z P, TANG Y. Layered niobic acid with self-exfoliatable nanosheets and adjustable acidity for catalytic hydration of ethylene oxide[J]. J Catal, 2011, 280(2):247-254. doi: 10.1016/j.jcat.2011.03.026 [19] TURCO R, ARONNE A, CARNITI P, GERVASINI A, MINIERI L, PERNICE P, TESSER R, VITIELLO R, DI SERIO M. Influence of preparation methods and structure of niobium oxide-based catalysts in the epoxidation reaction[J]. Catal Today, 2015, 254:99-103. doi: 10.1016/j.cattod.2014.11.033 [20] SRILATHA K, LINGAIAH N, DEVI BLAP, PRASAD RBN, VENKATESWAR S, PRASAD PSS. Esterification of free fatty acids for biodiesel production over heteropoly tungstate supported on niobia catalysts[J]. Appl Catal A:Gen, 2009, 365(1):28-33. doi: 10.1016/j.apcata.2009.05.025 [21] DE LA CRUZ M H C, ABDEL-REHIM M A, ROCHA A S, DA SILVA J F C, DA COSTA FARO JR A, LACHTER E R. Liquid phase alkylation of anisole by benzyl alcohol catalyzed on alumina-supported niobia[J]. Catal Commun, 2007, 8(11):1650-1654. doi: 10.1016/j.catcom.2007.01.019 [22] WANG F, WU H Z, LIU C L, YANG R Z, DONG W S. Catalytic dehydration of fructose to 5-hydroxymethylfurfural over Nb2O5 catalyst in organic solvent[J]. Carbohydr Res, 2013, 368:78-83. doi: 10.1016/j.carres.2012.12.021 [23] NGEE E L S, GAO Y, CHEN X, LEE T M, HU Z, ZHAO D, YAN N. Sulfated mesoporous niobium oxide catalyzed 5-hydroxymethylfurfural formation from sugars[J]. Ind Eng Chem Res, 2014, 53(37):14225-14233. doi: 10.1021/ie501980t [24] GAO J L, GAO S, LIU C L, LIU Z T, DONG W S. Synthesis, characterization, and catalytic application of ordered mesoporous carbon-niobium oxide composites[J]. Mate Res Bull, 2014, 59(16):131-136. http://www.sciencedirect.com/science/article/pii/S0025540814003729 [25] GARCÍA-SANCHO C, AGIRREZABAL-TELLERIA I, GVEMEZ MB, MAIRELES-TORRES P. Dehydration of d-xylose to furfural using different supported niobia catalysts[J]. Appl Catal B:Environ, 2014, 152-153:1-10. doi: 10.1016/j.apcatb.2014.01.013 [26] ZHAO D, FENG J, HUO Q, MELOSH N, FREDRICKSON G H, CHMELKA B F, STUCKY G D. Triblock copolymer syntheses of mesoporous silica with periodic 50 to 300 angstrom pores[J]. Science, 1998, 279(5350):548-552. doi: 10.1126/science.279.5350.548 [27] 李春晶, 沈健, 张亮, 王超.介孔分子筛Nb2O5/SBA-15催化合成油酸甲酯[J].辽宁石油化工大学学报, 2008, 28(4):9-15. http://en.cnki.com.cn/Article_en/CJFDTotal-FSSX200804002.htmLI Chun-jing, SHEN Jian, ZHANG Liang, WANG Chao. Catalytic synthesis of methyloleate by mesoporous sieve Nb2O5/SBA-15[J]. J Liaoning Univ Pet Chem Technol, 2008, 28(4):9-15. http://en.cnki.com.cn/Article_en/CJFDTotal-FSSX200804002.htm [28] ZHANG Y, WANG J, LI X, LIU X, XIA Y, HU B, LU G, WANG Y. Direct conversion of biomass-derived carbohydrates to 5-hydroxymethylfurural over water-tolerant niobium-based catalysts[J]. Fuel, 2015, 139:301-307. doi: 10.1016/j.fuel.2014.08.047 [29] ORDOMSKY V V, SUSHKEVICH V L, SCHOUTEN J C, VAN DER SCHAAF J, NIJHUIS T A. Glucose dehydration to 5-hydroxymethylfurfural over phosphate catalysts[J]. J Catal, 2013, 300(3):37-46. http://www.sciencedirect.com/science/article/pii/S0021951712004216 [30] HAFIZI H, NAJAFI CHERMAHINI A, SARAJI M, MOHAMMADNEZHAD G. The catalytic conversion of fructose into 5-hydroxymethylfurfural over acid-functionalized KIT-6, an ordered mesoporous silica[J]. Chem Eng J, 2016, 294:380-388. doi: 10.1016/j.cej.2016.02.082 [31] KUO C H, POYRAZ A S, JIN L, MENG Y, PAHALAGEDARA L, CHEN S Y, KRIZ DA, GUILD C, GUDZ A, SUIB S L. Heterogeneous acidic TiO2 nanoparticles for efficient conversion of biomass derived carbohydrates[J]. Green Chem, 2014, 16(2):785-791. doi: 10.1039/c3gc40909k [32] 刘彦丽, 王福余, 王崇, 赵振波.固体酸WO3/ZrO2催化果糖脱水合成5-羟甲基糠醛[J].化工进展, 2014, 33(1):105-109. http://d.wanfangdata.com.cn/Periodical/hgjz201401018LIU Yan-li, WANG Fu-yu, WANG Chong, ZHAO Zhen-bo. WO3/ZrO2 for fructose dehydration to 5-hydrocymethylfurfural as a solid acid catalyst[J]. Chem Int Eng Prog, 2014, 33(1):105-109. http://d.wanfangdata.com.cn/Periodical/hgjz201401018 [33] 王建刚, 张云云, 王勇, 朱丽伟, 崔洪友, 易维明.分级有序多孔磺化碳催化果糖转化制5-羟甲基糠醛[J].燃料化学学报, 2016, 44(11):1341-1347. doi: 10.3969/j.issn.0253-2409.2016.11.010WANG Jian-gang, ZHANG Yun-yun, WANG Yong, ZHU Li-wei, CUI Hong-you, YI Wei-ming. Catalytic fructose dehydration to 5-hydroxymethylfurfural over sulfonated carbons with hierarchically ordered pores[J]. J Fuel Chem Technol, 2016, 44(11):1341-1347. doi: 10.3969/j.issn.0253-2409.2016.11.010 -





 下载:
下载: