First-principle study on the reaction mechanism of water-gas shift on the Fe3O4 (001)-B surface
-
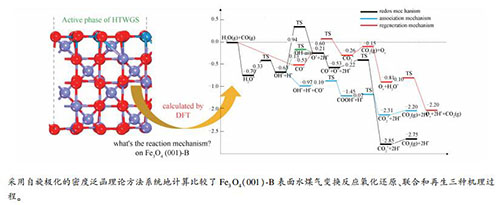
摘要: 采用自旋极化的密度泛函理论方法系统地研究了Fe3O4(001)-B表面水煤气变换的反应机理,计算了整个反应历程。结果表明,对于Fe3O4(001)-B表面上的水煤气变换反应,氧化还原、联合和再生三种反应路径共存,但氧化还原和联合机理的有效能垒较低,因而更占优势。对于生成H2的基元反应,其活性受表面H浓度和催化剂表面O缺陷浓度影响;较高的表面H浓度和O缺陷浓度均有利于H2生成。这些结果有助于进一步认识铁氧催化剂上的水煤气变换反应机理。
-
关键词:
- 水煤气变换反应 /
- Fe3O4 (001)-B表面 /
- 反应机理 /
- 第一性原理
Abstract: The reaction mechanism of water-gas shift (WGS) on the Fe3O4 (001)-B surface was systematically studied by using the density functional theory (DFT) calculation with spin polarization. The results show that for the WGS on the Fe3O4 (001)-B surface, three reaction routes including redox, association and regeneration ones coexist, though the redox and association routes may be more important with much lower effective energy barriers. The elementary reaction of H2 formation is influenced by the concentrations of surface H and O defects; higher concentrations of H species and O defects on the catalyst surface are beneficial to the formation of H2. These results should be helpful for a better understanding of the WGS reaction mechanism on the iron-oxygen catalyst.-
Key words:
- water-gas shift reaction /
- Fe3O4 (001)-B /
- reaction mechanism /
- first principle
-
图 2 吸附的H2O、CO、CO2和H2的最稳定构型及吸附能
(red ball for surface O, pink ball for O of H2O or CO2, light-blue ball for subsurface Fe, black-blue ball for surface Fe, white ball for H, black ball for C)
Figure 2 Most stable adsorption structures and adsorption energies (eV) of H2, CO, CO2 and H2 on Fe3O4 (001)-B surface
表 1 H与Fe3O4 (001)-B表面作用后的Bader电荷转移分析
Table 1 Bader charge analysis of H interacted with different sites of Fe3O4 (001)-B
Fe Oa Ob △e 0.352 -0.667 -0.695 -
[1] RHODES, C, HUTCHINGS G J, WARD A M. Water-gas shift reaction:Finding the mechanistic boundary[J]. Catal Today, 1995, 23:43-58. doi: 10.1016/0920-5861(94)00135-O [2] NEWSOME D S. The water-gas shift reaction[J]. Catal Rev, 1980, 21:275-318. doi: 10.1080/03602458008067535 [3] O'BRIEN R J, XU L, SPICER R L, BAO S, MILBURN D R, DAVIS B H. Activity and selectivity of precipitated iron Fischer-Tropsch catalysts[J]. Catal Today, 1997, 36:325-334. doi: 10.1016/S0920-5861(96)00246-5 [4] ANDREEV A, IDAKIEV V, MIHAJLOVA D, SHOPOV D. Iron-based catalysts for the water-gas shift reaction promoted by first-row transition metal oxides[J]. Appl Catal, 1986, 22:385-387. doi: 10.1016/S0166-9834(00)82645-7 [5] RHODES C, WILLIAMS B P, KING F. Promotion of Fe3O4/Cr2O3 high temperature water gas shift catalyst[J]. Catal Commun, 2002, 3:381-384. doi: 10.1016/S1566-7367(02)00156-5 [6] KUNDU M L, SENGUPTA A C, MAITI G C. Characterization of chromia-promoted γ-iron oxide catalysts and their CO conversion efficiency[J]. J Catal, 1988, 112:375-383. doi: 10.1016/0021-9517(88)90151-0 [7] PATLOLLA A, CARINO E V, EHRLICH S N. Application of operando XAS, XRD, and Raman spectroscopy for phase speciation in water gas shift reaction catalysts[J]. Acs Catal, 2012, 2:2216-2223. doi: 10.1021/cs300414c [8] PATLOLLA A, CARINO E V, EHRLICH S N, STAVITSKI E, FRENKEL A I. Application of operando XAS, XRD, and Raman spectroscopy for phase speciation in water gas shift reaction catalysts[J]. ACS Catal, 2012, 2:2216-2223. doi: 10.1021/cs300414c [9] REDDY G K, BOOLCHAND P, SMIRNIOTIS P G. Unexpected behavior of copper in modified ferrites during high temperature WGS reaction aspects of Fe3+ Fe2+ redox chemistry from Mössbauer and XPS studies[J]. J Phys Chem C, 2012, 116:11019-11031. doi: 10.1021/jp301090d [10] CHERKEZOVA-ZHELEVA Z, MITOV I. In situ Mössbauer investigation of iron oxide catalyst in water gas shift reaction-impact of oxyreduction potential and temperature[J]. J Phys Conf Ser, 2010, 217:012044. doi: 10.1088/1742-6596/217/1/012044 [11] GRILLO M E, FINNIS M W, RANKE W. Surface structure and water adsorption on Fe3O4 (111):Spin-density functional theory and on-site Coulomb interactions[J]. Phys Rev B, 2008, 77:075407. doi: 10.1103/PhysRevB.77.075407 [12] HUANG D M, CAO D B, LI Y W, JIAO H J. Density function theory study of CO adsorption on Fe3O4 (111) surface[J]. J Phys Chem B, 2006, 110:13920-13925. doi: 10.1021/jp0568273 [13] CHEN L, NI G, HAN B, ZHOU C G, WU J P. Mechanism of water gas shift reaction on Fe3O4 (111) Surface[J]. Acta Chim Sin, 2011, 69:393-398. http://d.old.wanfangdata.com.cn/Periodical/hxxb201104005 [14] HUANG L, HAN B, ZHANG Q F, FAN M H, CHENG H S. Mechanistic study on water gas shift reaction on the Fe3O4 (111) reconstructed surface[J]. J Phys Chem C, 2015, 119:28934-28945. doi: 10.1021/acs.jpcc.5b09192 [15] RIM K T, EOM D, CHAN S W. Scanning tunneling microscopy and theoretical study of water adsorption on Fe3O4:Implications for catalysis[J]. J Am Chem Soc, 2012, 134:18979-18985. doi: 10.1021/ja305294x [16] WANG G C, JIANG L, CAI Z S, PAN Y, ZHAO X, HUANG W, XIE K, LI Y, SUN Y, ZHONG B. Surface structure sensitivity of the water-gas shift reaction on Cu(hkl) surfaces:A theoretical study[J]. J Phys Chem B, 2003, 107(2):557-562. doi: 10.1021/jp0215567 [17] WANG G C, NAKAMURA J. Structure sensitivity for forward and reverse water-gas shift reactions on copper surfaces:A DFT study[J]. J Phys Chem Lett, 2010, 1(20):3053-3057. doi: 10.1021/jz101150w [18] WANG, Y X, WANG G C. A systematic theoretical study of water gas shift reaction on Cu(111) and Cu(110):Potassium effect[J]. ACS Catal, 2019, 9:2261-2274. doi: 10.1021/acscatal.8b04427 [19] PARKINSON G S, MANZ T A, NOVOTNY Z, SPRUNGER P T, DIEBOLD U. Antiphase domain boundaries at the Fe3O4 (001) surface[J]. Phy Rev B, 2012, 85(19):195451-195457. doi: 10.1103/PhysRevB.85.195451 [20] PENTCHEVA R, MORITZ W, RUNDGREN J, FRANK S, SCHRUPP D, SCHEFFLER M. A combined DFT/Leed-approach for complex oxide surface structure determination:Fe3O4(001)[J]. Surf Sci, 2008, 602(7):1299-1305. doi: 10.1016/j.susc.2008.01.006 [21] NOVOTNY Z, MULAKALURI N, EDES Z, SCHMID M, PENTCHEVA R, DIEBOLD U. Probing the surface phase diagram of Fe3O4(001) towards the fe-rich limit:Evidence for progressive reduction of the surface[J]. Phys Rev B, 2013, 87(19):2329-2337. https://www.researchgate.net/publication/240918793_Probing_the_surface_phase_diagram_of_Fe3O4001_towards_the_Fe_rich_limit_Evidence_for_progressive_reduction_of_the_surface?ev=auth_pub [22] KRESSE G, FURTHMÜLLER J. Efficiency of Ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set[J]. Comput Mater Sci, 1996, 6:15-50. doi: 10.1016/0927-0256(96)00008-0 [23] KRESSE G, FURTHMÜLLER J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set[J]. Phys Rev B, 1996, 54:11169-11186. doi: 10.1103/PhysRevB.54.11169 [24] BLÖCHL P E. Projector augmented-wave method[J]. Phys Rev B, 1994, 50:17953-17979. doi: 10.1103/PhysRevB.50.17953 [25] KRESSE G, HAFNER J. First-principles study of the adsorption of atomic H on Ni (111), (100) and (110)[J]. Surf Sci, 2000, 459:287-302. doi: 10.1016/S0039-6028(00)00457-X [26] PERDEW J P, BURKE K, ERNZERHOF M. Generalized gradient approximation made simple[J]. Phys Rev Lett, 1996, 77:3865- 3868. doi: 10.1103/PhysRevLett.77.3865 [27] PERDEW J P, BURKE K, ERNZERHOF M. Generalized gradient approximation made simple[J]. Phys Rev Lett, 1997, 78:1396. http://d.old.wanfangdata.com.cn/NSTLQK/NSTL_QKJJ0212186302/ [28] METHFESSEL M, PAXTON A T. High-precision sampling for Brillouin-zone integration in metals[J]. Phys Rev B, 1989, 40:3616. doi: 10.1103/PhysRevB.40.3616 [29] JÓNSSON H, MILLS G, JACOBSEN K W. In Classical and Quantum Dynamics in Condensed Phase Simulations[M]. Singapore:World Scientific, 1998:385. [30] HENKELMANN G, UBERUAGA B P, JNSSON H. A climbing image nudged elastic band method for finding saddle points and minimum energy paths[J]. J Chem Phys, 2000, 113:9901-9904. doi: 10.1063/1.1329672 [31] REDDY G K, SMIRNIOTIS P G. Effect of copper as a dopant on the water gas shift activity of Fe/Ce and Fe/Cr modified ferrites[J]. Catal Lett, 2011, 141:27-32. doi: 10.1007/s10562-010-0465-2 [32] HENKELMAN G, ARNALDSSON A, JÓNSSON H. A fast and robust algorithm for Bader decomposition of charge density[J]. Comput Mater Sci, 2006, 36(3):0-360. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=8b349feb49fca205cfb22ce02f8e04cf -





 下载:
下载: