Construction of synergistic and efficient iron-based catalysts for hydrogenation of CO2 to higher hydrocarbons
-
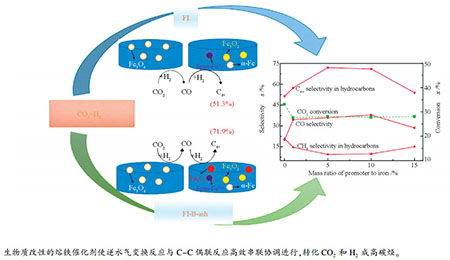
摘要: 采用物理掺杂法制备了生物质灰分作为助剂的融铁催化剂,通过X射线衍射、透射电镜、穆斯堡尔谱等方法对催化剂进行了表征,并在固定床反应器中对其CO2加氢制高碳烃的催化性能进行了评价。结果表明,与不含生物质灰分助剂的催化剂相比,添加助剂的融铁催化剂粒径较小且尺寸分布较窄,Fe3O4、Fe5C2、Fe3C和α-Fe四相协同共存,进而促使逆水气变换反应与C-C偶联的串联反应高效进行,在有效抑制甲烷生成的同时,可明显提升高碳烃选择性。高碳烃产物以C4-18的烃类为主,在300 ℃、1.0 MPa、4800 h-1、H2/CO2=3.0、助剂添加量为5%(质量分数)的条件下,其在烃类产物中选择性最高可达73.9%。Abstract: A series of fused iron (FI) catalysts promoted with biomass ash were prepared by physical mixing method and characterized by X-ray diffraction, transmission electron microscopy and Mossbauer spectroscopy. The catalytic performance of CO2 hydrogenation to higher hydrocarbons was evaluated in a fixed bed reactor. The results show that compared with the catalyst without biomass ash (B-ash), the fused iron catalysts promoted with biomass ash have smaller particle size and narrower size distribution, and the four phases of Fe3O4, Fe5C2, Fe3C as well as α-Fe coexist in synergy. Thus, the tandem reaction of reverse water gas shift (RWGS) and C-C coupling proceed efficiently, and the selectivity of higher hydrocarbons is significantly improved while methane formation is effectively suppressed. Among the products, C4-18 hydrocarbons are dominant. The C4-18 hydrocarbons' selectivity in all hydrocarbons reaches 73.9% at the conditions of 300℃, 1.0 MPa, 4800 h-1, H2/CO2=3.0 as well as the additive amount of the promoter is 5% (mass ratio).
-
Key words:
- CO2 hydrogenation /
- high hydrocarbons /
- Fe-based catalyst /
- biomass /
- synergism
-
表 1 B-ash元素组成及含量
Table 1 The elemental composition of calcined corncob
Material Composition of element w/% O K Si Ca Cl Al Fe Mg P Na S others B-ash 26.9 22.8 21.8 7.2 6.8 5.6 3.2 2.1 1.0 0.9 0.8 0.9 表 2 不同催化剂的比表面积
Table 2 Specific surface area of different catalysts
Catalyst FI FI-1-B-ash FI-5-B-ash FI-10-B-ash FI-15-B-ash Surface area A /(m2·g-1) 4.3 1.7 1.7 1.0 1.4 表 3 空速对CO2加氢性能的影响
Table 3 Effect of velocity on the performance of CO2 hydrogenation
Velocity /h-1 CO2 conv. x/% CO sel. s/% Hydrocarbons sel. s/% CH4 C2, 3 C4+ 2400 44.4 11.86 9.9 19.1 71.0 4800 28.1 35.9 9.5 18.6 71.9 9600 29.8 28.0 10.6 19.4 70.0 reaction conditions: H2/CO2=3.0, t=320 ℃, p=1.0 MPa 表 4 压力对CO2加氢性能的影响
Table 4 Effect of pressure on the performance of CO2 hydrogenation
Pressure p/MPa CO2 conv. x/% CO sel. s/% Hydrocarbons sel. s/% CH4 C2, 3 C4+ 0.5 27.0 45.3 9.4 19.4 71.2 1.0 28.1 35.9 9.5 18.6 71.9 2.0 38.7 13.8 10.7 18.8 70.5 reaction conditions: H2/CO2=3.0, t=320 ℃, v=4800 h-1 表 5 温度对CO2加氢性能的影响
Table 5 Effect of temperature on the performance of CO2 hydrogenation
Temperature t/℃ CO2 conv. x/% CO sel. s/% Hydrocarbons sel. s/% CH4 C2, 3 C4+ 280 21.0 34.4 9.9 17.1 73.0 300 23.8 37.8 8.1 18.0 73.9 320 28.1 35.9 9.5 18.6 71.9 340 32.8 31.6 12.6 21.0 66.4 reaction conditions: H2/CO2=3.0, p=1.0 MPa, v=4800 h-1 -
[1] DATTAS J, KHUMNOONC, LEEZ H, MOON W K, DOCAO S, NGUYEN T H, HWANG I C, MOON D, OLEYNIKOV P, TERASAKI O, YOON K B. CO2 capture from humid flue gases and humid atmosphere using a microporous coppersilicate[J]. Science, 2015, 350(6258):302-306. doi: 10.1126/science.aab1680 [2] WANG W, WANG S P, MA X B, GONG J L. Recent advances in catalytic hydrogenation of carbon dioxide[J]. Chem Soc Rev, 2011, 40(7):3703-3727. doi: 10.1039/c1cs15008a [3] DORNER R W, HARDY D R, WILLIAMS F W, WILLAUER H D. Heterogeneous catalytic CO2 conversion to value-added hydrocarbons[J]. Energy Environ Sci, 2010, 3(7):884-890. doi: 10.1039/c001514h [4] ARAKAWA H, ARESTA M, ARMOR J N, BARTEAU M A, BECKMAN E J, BELL A T, BERCAW J E, CREUTZ C, DINJUS E, DIXON D A, DOMEN K, DUBOIS D L, ECKERT J, FUJITA E, GIBSON D H, GODDARD W A, GOODMAN D W, KELLER J, KUBAS G J, KUNG H H, LYONS J E, MANZER L E, MARKS T J, MOROKUMA K, NICHOLAS K M, PERIANA R, QUE L, ROSTRUP-NIELSON J, SACHTLER W M H, SCHMIDT L D, SEN A, SOMORJAI G A, STAIR P C, STULTS B R, TUMAS W. Catalysis research of relevance to carbon management progress, challenges, and opportunities[J]. Chem Rev, 2001, 101(4):953-996. doi: 10.1021/cr000018s [5] DIMITRIOU I, GARCÍA-GUTIÉRREZ P, ELDER R H, CUÉLLAR-FRANCA R M, AZAPAGIC A, ALLEN R W K. Carbon dioxide utilisation for production of transport fuels:Process and economic analysis[J]. Energy Environ Sci, 2015, 8(6):1775-1789. doi: 10.1039/C4EE04117H [6] ROY S C, VARGHESE O K, PAULOSE M, GRIMES C A. Toward solar fuels:Photocatalytic conversion of carbon dioxide to hydrocarbons[J]. ACS Nano, 2010, 4(3):1259-1278. doi: 10.1021/nn9015423 [7] LI W H, WANG H Z, JIANG X, ZHU J, LIU Z M, GUO X W, SONG C S. A short review of recent advances in CO2 hydrogenation to hydrocarbons over heterogeneous catalysts[J]. Rsc Adv, 2018, 8(14):7651-7669. doi: 10.1039/C7RA13546G [8] CENTI G, QUADRELLI E A, PERATHONER S. Catalysis for CO2 conversion:A key technology for rapid introduction of renewable energy in the value chain of chemical industries[J]. Energy Environ Sci, 2013, 6(6):1711-1731. doi: 10.1039/c3ee00056g [9] YANG H Y, ZHANG C, GAO P, WANG H, LI X P, ZHONG L S, WEI W, SUN Y H. A review of the catalytic hydrogenation of carbon dioxide into value-added hydrocarbons[J]. Catal Sci Technol, 2017, 7(20):4580-4598. doi: 10.1039/C7CY01403A [10] WEI J, GE Q J, YAO R W, WEN Z Y, FANG C Y, GUO L S, XU H Y, SUN J. Directly converting CO2 into a gasoline fuel[J]. Nat Commun, 2017, (8):15174. https://www.researchgate.net/publication/314238125_Directly_Converting_CO2_into_a_Gasoline_Fuel [11] GAO P, LI S G, BU X N, DANG S S, LIU Z Y, WANG H, ZHONG L S, QIU M H, YANG C G, CAI J, WEI W, SUN Y H. Direct conversion of CO2 into liquid fuels with high selectivity over a bifunctional catalyst[J]. Nat Chem, 2017, 9(10):1019-1024. doi: 10.1038/nchem.2794 [12] LIU J H, ZHANG A F, JIANG X, LIU M, SUN Y W, SONG C S, GUO X W. Selective CO2 hydrogenation to hydrocarbons on Cu-promoted Fe-based catalysts:Dependence on Cu-Fe interaction[J]. ACS Sustainable Chem Eng, 2018, 6(8):10182-10190. doi: 10.1021/acssuschemeng.8b01491 [13] NI Y M, CHEN Z Y, FU Y, LIU Y, ZHU W L, LIU Z M. Selective conversion of CO2 and H2 into aromatics[J]. Nat Commun, 2018, 9(1), 3457. doi: 10.1038/s41467-018-05880-4 [14] NIEMELÄ, NOKKOSMÄKI M. Activation of carbon dioxide on Fe-catalysts[J]. Catal Today, 2005, 100(3/4):269-274. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=0c637ec2e4b63702c34524c4220551bb [15] GUO L S, SUN J, JI X W, WEN Z Y, YAO R W, XU H Y, GE Q J. Directly converting carbon dioxide to linear α-olefins on bio-promoted catalysts[J]. Commun Chem, 2018, 1(1). [16] ZHANG J L, LU S P, SU X J, FAN S B, MA Q X, ZHAO T S. Selective formation of light olefins from CO2hydrogenation over Fe-Zn-K catalysts[J]. J CO2 Util, 2015, 12:95-100. doi: 10.1016/j.jcou.2015.05.004 [17] YOU Z Y, DENG W P, ZHANG Q H, WANG Y. Hydrogenation of carbon dioxide to light olefins over non-supported iron catalyst[J]. Chin J Catal, 2013, 34(5):956-963. doi: 10.1016/S1872-2067(12)60559-2 [18] SUN B, XU K, LUAN N, QIAO M H, FRANKLIN (FENG) T. Preparation and catalysis of carbon-supported iron catalysts for Fischer-Tropsch synthesis[J]. ChemCatChem, 2012, 4(10):1498-1511. doi: 10.1002/cctc.v4.10 [19] YAN S R, JUN K W, HONG J S, CHOI M J, LEE K W. Promotion effect of Fe-Cu catalyst for the hydrogenation of CO2 and application to slurry reactor[J]. Appl Catal A:Gen, 2000, 194/195:63-70. doi: 10.1016/S0926-860X(99)00354-3 [20] GUO L S, SUN J, WEI J, WEN Z Y, XU H Y, GE Q J. Fischer-Tropsch synthesis over iron catalysts with corncob-derived promoters[J]. Energy Chem, 2017, 26(4):632-638. doi: 10.1016/j.jechem.2017.03.017 [21] SUN J, XU H Y, LIU G G, ZHU P F, FAN R G, YONEYAMA Y, TSUBAKI N. Green synthesis of rice bran microsphere catalysts containing natural biopromoters[J]. ChemCatChem, 2015, 7(11):1642-1645. doi: 10.1002/cctc.v7.11 [22] 廖小元.铁基催化剂活性相Fe3C表面的F-T反应机理的量子化学研究[D].太原: 中国科学院山西煤炭化学研究所, 2007. http://www.irgrid.ac.cn/handle/1471x/120172?mode=full&submit_simple=Show+full+item+recordLIAO Xiao-yuan. Theoretical Study of F-T Reaction Mechanism on Fe3C surfaces[D]. Taiyuan: Shanxi Institute of Coal Chemistry, Chinese Academy of Sciences, 2007. http://www.irgrid.ac.cn/handle/1471x/120172?mode=full&submit_simple=Show+full+item+record [23] 陈运红. CO在Fe(111)和Fe(100)面上吸附的量子化学研究[D].广州: 暨南大学, 2005. http://cdmd.cnki.com.cn/Article/CDMD-10559-2005141363.htmCHEN Yun-hong. Quantum chemical study of CO adsorption on the Fe(111) and Fe(100) surfaces[D]. Guangzhou: Jinan University, 2005. http://cdmd.cnki.com.cn/Article/CDMD-10559-2005141363.htm [24] GNANAMANI M K, HAMDEH H H, JACOBS G, SHAFER W D, SPARKS D E, KEOGH R A, DAVIS B H. Fischer-Tropsch synthesis:Activity and selectivity of chi-Fe5C2 and circle minus-Fe3C carbides[J]. Abstracts of Papers of the American Chemical Society, 2014, 248. [25] 位健, 马现刚, 方传艳, 葛庆杰, 徐恒泳. Fe/SiO2纳米复合物上合成气制低碳烯烃催化性能研究[J].燃料化学学报, 2014, 42(7):827-832. http://www.ccspublishing.org.cn/article/id/100033165WEI Jian, MA Xian-gang, FANG Chuan-yan, GE Qing-jie, XU Heng-yong. Iron-silica nanocomposites as a catalyst for the selective conversion of syngas to light olefins[J]. J Fuel Chem Technol, 2014, 42(7):827-832. http://www.ccspublishing.org.cn/article/id/100033165 [26] POUR A N, SHAHRI S M K, BOZORGZADEH H R, ZAMANI Y, TAVASOLI A, MARVAST M A. Effect of Mg, La and Ca promoters on the structure and catalytic behavior of iron-based catalysts in Fischer-Tropsch synthesis[J]. Appl Catal A:Gen, 2008, 348:201-208. doi: 10.1016/j.apcata.2008.06.045 [27] SATTHAWONG R, KOIZUMI N, SONG C S, PRASASSARAKICH P. Light olefin synthesis from CO2 hydrogenation over K-promoted Fe-Co bimetallic catalysts[J]. Catal Today, 2015, 251:34-40. doi: 10.1016/j.cattod.2015.01.011 [28] GALVIS H M T, BITTER J H, KHARE C B, RUITENBEEK M, DUGULAN A I, DE JONG K P. Supported iron nanoparticles as catalysts for sustainable production of lower olefins[J]. Science, 2012, 335:835-838. doi: 10.1126/science.1215614 [29] YANG Y, XIANG H W, XU Y Y, BAI L, LI Y W. Effect of potassium promoter on precipitated iron-manganese catalyst for Fischer-Tropsch synthesis[J]. Appl Catal A:Gen, 2004, 266(2):181-194. doi: 10.1016/j.apcata.2004.02.018 [30] SATTHAWONG R, KOIZUMI N, SONG C S, PRASASSARAKICH P. Light olefin synthesis from CO2hydrogenation over K-promoted Fe-Co bimetallic catalysts[J]. Catal Today, 2015, 251:34-40. doi: 10.1016/j.cattod.2015.01.011 -





 下载:
下载: