Preparation of platinum-silver alloy nanoparticles and their catalytic performance in methanol electro-oxidation
-
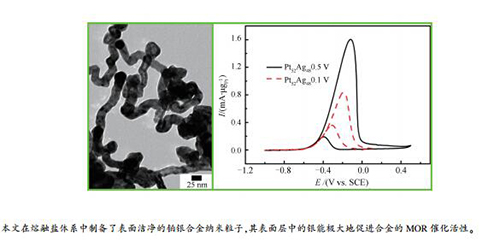
摘要: 采用一种无需使用任何有机表面活性剂或溶剂的方法,在熔融盐体系中制备了铂银纳米合金颗粒,考察了合金中元素银对碱性电解质中甲醇电氧化反应(MOR)的催化作用。透射电子显微镜表征结果显示,当前躯体铂银物质的量比为1时,可以得到组成为Pt52Ag48的合金纳米管。甲醇电氧化反应测试结果表明,具有干净表面的Pt52Ag48纳米管比常规的Pt黑具有更好的催化性能。Pt52Ag48合金纳米管的催化活性与其最大正扫电位密切相关,正扫电位从-1.0到0.5 V(vs.SCE),MOR峰值电流达到1.61 mA/μgPt,是从-1.0到0.1 V(vs.SCE)正扫电位的1.92倍。铂银合金表面层中的Ag元素主要通过在电化学循环中发生氧化还原反应来促进合金的MOR活性。研究结果可以为铂银合金在直接甲醇燃料电池(DMFC)中的应用提供理论支持。Abstract: Platinum-silver alloy nanoparticles (PtxAgy NPs) were synthesized in a molten salt system without using any organic surfactants or solvents; the catalytic role of Ag in the methanol electrooxidation reaction (MOR) in alkaline electrolyte over PtxAgy NPs was investigated. The TEM images suggest that Pt52Ag48 nanotubes (NTs) can be obtained when the Pt/Ag ratio in the molten salt precursor reaches 1. The methanol electrooxidation reaction test results indicate that the Pt52Ag48 NTs with a clean surface exhibits a much better catalytic performance than the conventional Pt black in MOR. Meanwhile, the catalytic activity of the Pt52Ag48 NTs is greatly related to the positive potential limit; the peak current of MOR reaches 1.61 mA/μgPt with a positive potential limit from -1.0 to 0.5 V (vs. SCE), which is 1.92 times higher than that with a positive potential limit from -1.0 to 0.1 V (vs. SCE). The Ag element in the surface layer of PtxAgy alloy may promote the MOR through a redox process during the electrochemical cycle. The insight shown in work should be beneficial to the application of PtxAgy alloy in the direct methanol fuel cells (DMFCs).
-
Figure 8 Cyclic voltammograms (a) and linear polarization curves (b) for MOR on the Pt black (JM), Pt11Ag89, Pt21Ag79, Pt52Ag48, Pt79Ag21 and Pt86Ag14 catalysts in the electrolyte of 0.5 moL/L KOH + 2 moL/L CH3OH, the insets in (a) and (b) show the corresponding activities at -0.25 V and the dependence between the onset potential of MOR and the catalysts composition, respectively (the scan rate is 20 mV/s)
Table 1 PtxAgy atomic molar ratios in the alloy NPs prepared with different Pt(NH3)4C2O4/CH3COOAg ratios in the molten salt precursor, analyzed by EDX
PtxAgy sample Pt(NH3)4C2O4/CH3COOAg Pt/Ag, by EDX Pt86Ag14 8:1 6.09 Pt79Ag21 4:1 3.89:1 Pt52Ag48 1:1 1.10:1 Pt21Ag79 1:4 1:3.88 Pt11Ag89 1:8 1:8.51 Table 2 MOR performance of Pt black, Pt11Ag89, Pt21Ag79, Pt52Ag48, Pt79Ag21 and Pt86Ag14 catalysts in 0.5 moL/L KOH + 2 moL/L CH3OH
Sample E0 /V Ep /V If /(mA·μgPt-1) Ib /(mA·μgPt-1) If/Ib I@-0.25V /(mA·μgPt-1) Pt black -0.77 -0.07 1.43 0.56 2.55 0.86 Pt11Ag89 -0.51 -0.28 0.02 - - 0.02 Pt21Ag79 -0.71 -0.25 0.34 0.03 11.33 0.33 Pt52Ag48 -0.91 -0.20 1.61 0.19 8.47 1.02 Pt79Ag21 -0.82 -0.21 0.43 0.06 7.17 0.39 Pt86Ag14 -0.66 -0.24 0.25 0.02 12.50 0.26 -
[1] SAXENA N, PRANEETH N, RAO K, PARIA S. Organization of palladium nanoparticles into fractal patterns for highly enhanced catalytic activity and anode material for direct borohydride fuel cells applications[J]. ACS Appl Energy Mater, 2018, 1(5):2164-2175. doi: 10.1021/acsaem.8b00211 [2] SHARAF O Z, ORHAN M F, An overview of fuel cell technology:Fundamentals and applications[J]. Renewable Sustainable Energ Rev, 2014, 32, 810-853. doi: 10.1016/j.rser.2014.01.012 [3] STAMENKOVIC V R, FOWLER B, MUN B S, WANG G, ROSS P N, LUCAS C A, MARKOVIĆ N M. Improved oxygen reduction activity on Pt3Ni (111) via increased surface site availability[J]. Science, 2007, 5811:493-497. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=3b77f1ddc321c7747e19a50cc90d6b82 [4] GASTEIGER H A, MARKOVIĆ N M, Just a dream-or future reality?[J]. Science, 2009, 324(5923):48-49. doi: 10.1126/science.1172083 [5] GASTEIGER H A, KOCHA S S, SOMPALLI B, WAGNER F T. Activity benchmarks and requirements for Pt, Pt-alloy, and non-Pt oxygen reduction catalysts for PEMFCs[J]. Appl Catal B:Environ, 2005, 56(1):9-35. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=089f3d5590acf0c14f937063006e430e [6] YANG H, DAI L, XU D, FANG J, ZOU S. Electrooxidation of methanol and formic acid on PtCu nanoparticles[J]. Electrochim Acta, 2010, 55(27):8000-8004. doi: 10.1016/j.electacta.2010.03.026 [7] CHEN J, LIM B, LEE E P, XIA Y. Shape-controlled synthesis of platinum nanocrystals for catalytic and electrocatalytic applications[J]. Nano Today, 2009, 4(1):81-95. doi: 10.1016/j.nantod.2008.09.002 [8] TIAN N, ZHOU Z Y, SUN S G, DING Y, WANG Z L. Synthesis of tetrahexahedral platinum nanocrystals with high-index facets and high electro-oxidation activity[J]. Science, 2007, 316(5825):732-735. doi: 10.1126/science.1140484 [9] HUANG X, ZHAO Z, FAN J, TAN Y, ZHENG N. Amine-assisted synthesis of concave polyhedral platinum nanocrystals having {411} high-index facets[J]. J Am Chem Soc, 2011, 133(13):4718-4721. doi: 10.1021/ja1117528 [10] TIAN N, ZHOU Z Y, SUN S G. Platinum metal catalysts of high-index surfaces:From single-crystal planes to electrochemically shape-controlled nanoparticles[J]. J Phys Chem C, 2008, 112(50):19801-19817. doi: 10.1021/jp804051e [11] LIM B, JIANG M, CAMARGO P H C, CHO E C, TAO J, LU X, ZHU Y, XIA Y. Pd-Pt bimetallic nanodendrites with high activity for oxygen reduction[J]. Science, 2009, 324(5932):1302-1305. doi: 10.1126/science.1170377 [12] XU D, LIU Z, YANG H, LIU Q, ZHANG J, FANG J, ZOU S, SUN K. Solution-based evolution and enhanced methanol oxidation activity of monodisperse platinum-copper nanocubes[J]. Angew Chem Int Ed, 2009, 48(23):4217-4221. doi: 10.1002/anie.200900293 [13] HONG F, SUN S, YOU H, YANG S, FANG J, GUO S, YANG Z, DING B, SONG X. Cu2O template strategy for the synthesis of structure-definable noble metal alloy mesocages[J]. Cryst Growth Des, 2011, 11(9):3694-3697. doi: 10.1021/cg2001893 [14] XU H, SONG P, WANG J, DU Y. Shape-controlled synthesis of platinum-copper nanocrystals for efficient liquid fuel electrocatalysis[J]. Langmuir, 2018, 34(27):7981-7988. doi: 10.1021/acs.langmuir.8b01729 [15] ZHAO H, YU C, YOU H, YANG S, GUO Y, DING B, SONG X. A green chemical approach for preparation of PtxCuy nanoparticles with a concave surface in molten salt for methanol and formic acid oxidation reactions[J]. J Mater Chem, 2012, 22(11):4780-4789. doi: 10.1039/c2jm15792f [16] ZHANG J, FANG J. A general strategy for preparation of Pt 3d-transition metal (Co, Fe, Ni) nanocubes[J]. J Am Chem Soc, 2009, 131(51):18543-18547. doi: 10.1021/ja908245r [17] WANG X X, HWANG S, PAN Y T, CHEN K, HE Y, KARAKALOS S, ZHANG H, SPENDELOW J S, SU D, WU G. Ordered Pt3Co intermetallic nanoparticles derived from metal-organic frameworks for oxygen reduction[J]. Nano Lett, 2018, 18(7):4163-4171. doi: 10.1021/acs.nanolett.8b00978 [18] ZHANG L, FISCHER J, JIA Y, YAN X, XU W, WANG X, CHEN J, YANG D, LIU H, ZHUANG L, HANKEL M, SEARLES D J, HUANG K, FENG S, BROWN C L, YAO X. Coordination of atomic Co-Pt coupling species at carbon defects as active sites for oxygen reduction reaction[J]. J Am Chem Soc, 2018, 140(34):10757-10763. doi: 10.1021/jacs.8b04647 [19] YANG D, YAN Z, LI B, HIGGINS D C, WANG J, LV H, CHEN Z, ZHANG C. Highly active and durable Pt-Co nanowire networks catalyst for the oxygen reduction reaction in PEMFCs[J]. Int J Hydrog Energy, 2016, 41(41):18592-18601. doi: 10.1016/j.ijhydene.2016.08.159 [20] DING J, BU L, GUO S, ZHAO Z, ZHU E, HUANG Y, HUANG X. Morphology and phase controlled construction of Pt-Ni nanostructures for efficient electrocatalysis[J]. Nano Lett, 2016, 16(4):2762-2767. doi: 10.1021/acs.nanolett.6b00471 [21] MATIN M A, JANG J H, KWON Y U. PdM nanoparticles (M=Ni, Co, Fe, Mn) with high activity and stability in formic acid oxidation synthesized by sonochemical reactions[J]. J Power Sources, 2014, 262:356-363. doi: 10.1016/j.jpowsour.2014.03.109 [22] YANG S, PENG Z, YANG H. Platinum lead nanostructures:Formation, phase behavior, and electrocatalytic properties[J]. Adv Funct Mater, 2008, 18(18):2745-2753. doi: 10.1002/adfm.200800266 [23] ZHAO H, LIU R, GUO Y, YANG S. Molten salt medium synthesis of wormlike platinum silver nanotubes without any organic surfactant or solvent for methanol and formic acid oxidation[J]. Phys Chem Chem Phys, 2015, 17(46):31170-31176. doi: 10.1039/C5CP05641A [24] CAO X, WANG N, HAN Y, GAO C, XU Y, LI M, SHAO Y. PtAg bimetallic nanowires:Facile synthesis and their use as excellent electrocatalysts toward low-cost fuel cells[J]. Nano Energy, 2015, 12:105-114. doi: 10.1016/j.nanoen.2014.12.020 [25] WISNIEWSKA J, YANG C, ZIOLEK M. Changes in bimetallic silver-platinum catalysts during activation and oxidation of methanol and propene[J]. Catal Today, 2019, 333:89-96. doi: 10.1016/j.cattod.2018.03.001 [26] LV J J, LI S S, ZHENG J N, WANG A J, CHEN J R, FENG J J. Facile synthesis of reduced graphene oxide supported PtAg nanoflowers and their enhanced electrocatalytic activity[J]. Int J Hydrog Energy, 2014, 39(7):3211-3218. doi: 10.1016/j.ijhydene.2013.12.112 [27] LI J, RONG H, TONG X, WANG P, CHEN T, WANG Z. Platinum-silver alloyed octahedral nanocrystals as electrocatalyst for methanol oxidation reaction[J]. J Colloid Interface Sci, 2018, 513:251-257. doi: 10.1016/j.jcis.2017.11.039 [28] LIU Q, HE Y M, WENG X, WANG A J, YUAN P X, FANG K M, FENG J J. One-pot aqueous fabrication of reduced graphene oxide supported porous PtAg alloy nanoflowers to greatly boost catalytic performances for oxygen reduction and hydrogen evolution[J]. J Colloid Interface Sci, 2018, 513:455-463. doi: 10.1016/j.jcis.2017.11.026 [29] STAMENKOVIC V R, MUN B S, ARENZ M, MAYRHOFER K J J, LUCAS C A, WANG G, ROSS P N, MARKOVIC N M. Trends in electrocatalysis on extended and nanoscale Pt-bimetallic alloy surfaces[J]. Nat Mater, 2007, 6:241. doi: 10.1038/nmat1840 [30] LIN R, CHE L, SHEN D, CAI X. High durability of Pt-Ni-Ir/C ternary catalyst of PEMFC by stepwise reduction synthesis[J]. Electrochim Acta, 2020, 330:135251. doi: 10.1016/j.electacta.2019.135251 [31] LIN R, CAI X, HAO Z, PU H, YAN H. Rapid microwave-assisted solvothermal synthesis of shape-controlled Pt-Ni alloy nanoparticles for PEMFC[J]. Electrochim Acta, 2018, 283:764-771. doi: 10.1016/j.electacta.2018.03.190 [32] JIANG Q, JIANG L, HOU H, QI J, WANG S, SUN G. Promoting effect of Ni in PtNi bimetallic electrocatalysts for the methanol oxidation reaction in alkaline media:Experimental and density functional theory studies[J]. J Phys Chem C, 2010, 114(46):19714-19722. doi: 10.1021/jp1039755 [33] WU F, ZHANG Z, ZHANG F, DUAN D, LI Y, WEI G, LIU S, YUAN Q, WANG E, HAO X. Exploring the role of cobalt in promoting the electroactivity of amorphous Ni-B nanoparticles toward methanol oxidation[J]. Electrochim Acta, 2018, 287:115-123. doi: 10.1016/j.electacta.2018.07.106 [34] PRABHURAM J, MANOHARAN R. Investigation of methanol oxidation on unsupported platinum electrodes in strong alkali and strong acid[J]. J Power Sources, 1998, 74(1):54-61. doi: 10.1016/S0378-7753(98)00012-3 [35] BLIZANAC B B, ROSS P N, MARKOVIC N M. Oxygen electroreduction on Ag(111):The pH effect[J]. Electrochim Acta, 2007, 52(6):2264-2271. doi: 10.1016/j.electacta.2006.06.047 [36] FENG L, GAO G, HUANG P, WANG X, ZHANG C, ZHANG J, GUO S, CUI D. Preparation of Pt Ag alloy nanoisland/graphene hybrid composites and its high stability and catalytic activity in methanol electro-oxidation[J]. Nanoscale Res Lett, 2011, 6:551. doi: 10.1186/1556-276X-6-551 [37] HE W, WU X, LIU J, ZHANG K, CHU W, FENG L, HU X, ZHOU W, XIE S. Formation of AgPt alloy nanoislands via chemical etching with tunable optical and catalytic properties[J]. Langmuir, 2010, 26:4443-4448. doi: 10.1021/la9034968 [38] FENG Y Y, BI L X, LIU Z H, KONG D S, YU Z Y. Significantly enhanced electrocatalytic activity for methanol electro-oxidation on Ag oxide-promoted PtAg/C catalysts in alkaline electrolyte[J]. J Catal, 2012, 290:18-25. doi: 10.1016/j.jcat.2012.02.013 [39] FENG Y Y, LIU Z H, KONG W Q, YIN Q Y, DU L X. Promotion of palladium catalysis by silver for ethanol electro-oxidation in alkaline electrolyte[J]. Int J Hydrog Energy, 2014, 39(6):2497-2504. doi: 10.1016/j.ijhydene.2013.12.004 [40] XU J B, ZHAO T S, LIANG Z X. Synthesis of active platinum-silver alloy electrocatalyst toward the formic acid oxidation reaction[J]. J Phys Chem C, 2008, 112(44):17362-17367. doi: 10.1021/jp8063933 [41] WU J, ZHANG J, PENG Z, YANG S, WAGNER F T, YANG H. Truncated octahedral Pt3Ni oxygen reduction reaction electrocatalysts[J]. J Am Chem Soc, 2010, 132(14):4984-4985. doi: 10.1021/ja100571h [42] CHEN X, WU G, CHEN J, CHEN X, XIE Z, WANG X. Synthesis of "clean" and well-dispersive Pd nanoparticles with excellent electrocatalytic property on graphene oxide[J]. J Am Chem Soc, 2011, 133(11):3693-3695. doi: 10.1021/ja110313d [43] MERGA G, SAUCEDO N, CASS L C, PUTHUSSERY J, MEISEL D. "Naked" gold nanoparticles:Synthesis, characterization, catalytic hydrogen evolution, and SERS[J]. J Phys Chem C, 2010, 114(35):14811-14818. doi: 10.1021/jp104922a [44] CASWELL K K, BENDER C M, MURPHY C J. Seedless, surfactantless wet chemical synthesis of silver nanowires[J]. Nano Lett, 2003, 3(5):667-669. doi: 10.1021/nl0341178 [45] SUN S H, YANG D Q, VILLERS D, ZHANG G X, SACHER E, DODELET J P. Template-and surfactant-free room temperature synthesis of self-assembled 3D Pt nanoflowers from single-crystal nanowires[J]. Adv Mater, 2008, 20(3):571-574. doi: 10.1002/adma.200701408 [46] HUANG C, JIANG J, LU M, SUN L, MELETIS E I, HAO Y. Capturing electrochemically evolved nanobubbles by electroless deposition. A facile route to the synthesis of hollow nanoparticles[J]. Nano Lett, 2009, 9(12):4297-4301. doi: 10.1021/nl902529y [47] ZHAO H, WU J, YOU H, YANG S, DING B, YANG Z, SONG X, YANG H. In situ chemical vapor reaction in molten salts for preparation of platinum nanosheets via bubble breakage[J]. J Mater Chem, 2012, 22(24):12046-12052. doi: 10.1039/c2jm31422c [48] ZHAO H, YANG S, YOU H, WU Y, DING B. Synthesis of surfactant-free Pt concave nanoparticles in a freshly-made or recycled molten salt[J]. Green Chem, 2012, 14(11):3197-3203. doi: 10.1039/c2gc35995b [49] PENG Z, YANG H. Ag-Pt alloy nanoparticles with the compositions in the miscibility gap[J]. J Solid State Chem, 2008, 181(7):1546-1551. doi: 10.1016/j.jssc.2008.03.013 [50] POUND B G, MACDONALD D D, TOMLINSON J W. The electrochemistry of silver in KOH at elevated temperatures-II. Cyclic voltammetry and galvanostatic charging studies[J]. Electrochim Acta, 1980, 25(5):563-573. doi: 10.1016/0013-4686(80)87058-7 [51] LIMA F, SANCHES C D, TICIANELLI E A. Physical characterization and electrochemical activity of bimetallic platinum-silver particles for oxygen reduction in alkaline electrolyte[J]. J Electrochem Soc, 2005, 152(7):1466-1473. doi: 10.1149/1.1933514 [52] XU C W, WANG H, SHEN P K, JIANG S P. Highly ordered Pd nanowire arrays as effective electrocatalysts for ethanol oxidation in direct alcohol fuel cells[J]. Adv Mater, 2007, 19(23):4256-4259. doi: 10.1002/adma.200602911 [53] FENG Y Y, ZHANG G R, MA J H, LIU G, XU B Q. Carbon-supported Pt/Ag nanostructures as cathode catalysts for oxygen reduction reaction[J]. Phys Chem Chem Phys, 2011, 13(9):3863-3872. doi: 10.1039/c0cp01612h [54] CHATENET M, GENIES B L, AUROUSSEAU M, DURAND R, ANDOLFATTO F. Oxygen reduction on silver catalysts in solutions containing various concentrations of sodium hydroxide-comparison with platinum[J]. J Appl Electrochem, 2002, 32(10):1131-1140. doi: 10.1023/A:1021231503922 [55] NAGLE L C, AHERN A J, BURKE D L. Some unusual features of the electrochemistry of silver in aqueous base[J]. J Solid State Electr, 2002, 6(5):320-330. doi: 10.1007/s100080100233 [56] JOVIC B M, JOVIC V D, STAFFORD G R. Cyclic voltammetry on Ag(111) and Ag(100) faces in sodium hydroxide solutions[J]. Electrochem Commun, 1999, 1(6):247-251. doi: 10.1016/S1388-2481(99)00049-1 [57] OROZCO G, PÉREZ M C, RINCÓ N A, GUTIÉRREZ C. Electrooxidation of methanol on silver in alkaline medium[J]. J Electroanal Chem, 2000, 495(1):71-78. http://www.sciencedirect.com/science/article/pii/S002207280000396X -





 下载:
下载: