Effect of composition of Cu-ZnO-CeO2 catalyst on its performance for methanol synthesis from CO2 hydrogenation
-
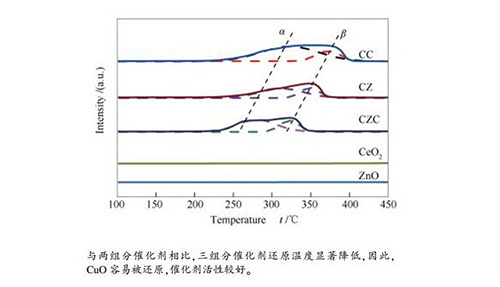
摘要: 采用并流沉淀法分别制备了CuO-CeO2(物质的量比为5:1)、CuO-ZnO(物质的量比为5:4)、CuO-ZnO-CeO2(物质的量比为5:4:1)三组目标催化剂,通过X射线衍射(XRD)、氢气升温还原(H2-TPR)、CO2程序升温脱附(CO2-TPD)、氮气吸附-脱附、X射线光电子能谱(XPS)、N2O滴定表征技术对催化剂的物化性能进行了测试,并在高温高压微催化反应器中对催化剂进行活性评价。研究了CuO-ZnO-CeO2组成对CO2加氢合成甲醇的影响。结果表明,与二组分催化剂相比较,三组分CuO-ZnO-CeO2催化剂物化性能及催化活性发生了很大变化,催化剂表面碱性位增强,热稳定性增强,CuO颗粒粒径变小,铜分散度以及氧空位浓度提高,最终催化活性显著提高。其中,CuO-ZnO-CeO2催化剂中,CuO颗粒粒径为8.2nm,铜的比表面积为68.4m2/g,铜分散度为7.19%,甲醇的选择性和收率分别为48.6%和0.057mmol/(g·min),催化剂活性较好。
-
关键词:
- CO2加氢反应 /
- CuO-ZnO-CeO2 /
- 催化剂 /
- 甲醇
Abstract: Three catalysts, CuO-CeO2 (5:1 molar ratio), CuO-ZnO (5:4 molar ratio) and CuO-ZnO-CeO2 (5:4:1 molar ratio), were prepared by coprecipitation method.The physicochemical properties of the catalysts were characterized by X-ray diffraction (XRD), hydrogen temperature reduction (H2-TPR), CO2 temperature programmed desorption (CO2-TPD), nitrogen adsorption desorption, X-ray photoelectron spectroscopy (XPS), N2O titration.The activity of the catalyst was evaluated in a catalytic microreactor.The results show that, the physicochemical properties and catalytic activity of the ternary CuO-ZnO-CeO2 catalyst are different from that of the binary catalyst, specifically, the strength of surface alkaline sites are increased, the thermal stability is enhanced, the particle size of CuO particles is reduced, the dispersion of copper and the concentration of oxygen vacancies are increased, and the catalytic activity is finally improved. In the CuO-ZnO-CeO2 catalyst, the CuO particle size is 8.2nm, the specific surface area of copper is 68.4m2/g, the copper dispersion is 7.19%, the selectivity and the yield of methanol are 48.6% and 0.057mmol/(g·min), respectively.-
Key words:
- CO2 hydrogenation /
- CuO-ZnO-CeO2 /
- catalyst /
- methanol
-
表 1 催化剂组成及结构参数
Table 1 Composition and structural parameters of the catalysts
Catalyst Composition of elemental w/% ABET
/(m2·g-1)ACu
/(m2·g-1)dCuO
/nmDCu
/%v
/(mL·g-1)Cu Zn Ce CC 83.3(53.4) - 16.7(46.6) 39.7 2.14 13.2 3.15 0.62 CZ 55.6(29.2) 44.4(70.8) - 44.2 2.96 10.4 3.87 0.75 CZC 50.0(42.8) 40.0(38.8) 10.0(18.4) 68.4 6.70 8.2 7.19 0.78 note: outer part of the bracket is composed of the body element and the parentheses are the surface element composition determined by XPS 表 2 催化剂XPS数据
Table 2 Catalyst XPS data
Catalyst Concentration percentage/% OⅡ/OⅠ Ce3+/Ce CC 1.00 11.08 CZ 6.96 - CZC 8.24 36.06 表 3 催化剂的活性评价
Table 3 Evaluation data of the catalysts
Catalyst xCO2/% sCH3OH/% YCH3OH/
(mmol·g-1·min-1)CC 13.9 23.8 0.012 CZ 18.0 34.7 0.039 CZC 26.5 48.6 0.057 reaction conditions: t=250 ℃, p=3 MPa, H2/CO2=3:1(volume ratio) and SV= 3000 mL/(g·h) -
[1] 唐宏青.煤化工工艺技术评述与展望Ⅰ.煤气化技术[J].燃料化学学报, 2001, 29(1):4-8. http://d.old.wanfangdata.com.cn/Periodical/rlhxxb200104001TANG Hong-qing. Review and prospect of coal chemical process technology I. Coal gasification technology[J]. J Fuel Chem Technol, 2001, 29(1):4-8. http://d.old.wanfangdata.com.cn/Periodical/rlhxxb200104001 [2] GENG W H, HAN H, LIU F, LIU X R, XIAO L F, WU W. N, P, S-codoped C@nano-Mo2C as an efficient catalyst for high selective synthesis of methanol from CO2 hydrogenation[J]. J CO2 Util, 2017, 21:64-71. doi: 10.1016/j.jcou.2017.06.016 [3] CARRADO K A, KIM J H, SONG C S, CASTAGNOLA N, MARSHALL C L, SCHWARTZ M M. HDS and deep HDS activity of CoMoS-mesostructured clay catalysts[J]. Catal Today, 2006, 116(4):478-484. http://www.sciencedirect.com/science/article/pii/S0920586106004135 [4] RAMACHANDRIYA K D, KUNDIYANA D K, WILKINS M R, TERRILL J B, ATIYEH H K, HUHNKE R L. Carbon dioxide conversion to fuels and chemicals using a hybrid green process[J]. Appl Energy, 2013, 112(4):289-299. http://www.sciencedirect.com/science/article/pii/S0306261913005242 [5] BAIKER A. Utilization of carbon dioxide in heterogeneous catalytic synthesis[J]. Appl Organomet Chem, 2000, 14(12):751-762. doi: 10.1002/1099-0739%28200012%2914%3A12%3C751%3A%3AAID-AOC85%3E3.0.CO%3B2-J [6] LI L, ZHANG Y, ZHENG Q, ZHENG Y H, CHEN C Q, SHE Y S, LIN X Y, WEI K M. Water-gas shift reaction over CuO/CeO2, catalysts:Effect of the thermal stability and oxygen vacancies of CeO2, supports previously prepared by different methods[J]. Catal Lett, 2009, 130(3/4):532-540. [7] TURSUNOV O, KUSTOV L, TILYABAEV Z. Methanol synthesis from the catalytic hydrogenation of CO2, over CuO-ZnO supported on aluminum and silicon oxides[J]. J Taiwan Inst Chem E, 2017, 78:416-422. [8] LAETITIA A, KILIAN K, LEIDY M, MARTINEZ T, YVAN Z, KSENIA P, ANNE C Iconography:Study of CuZnMOx oxides (M=Al, Zr, Ce, CeZr) for the catalytic hydrogenation of CO2 into methanol[J]. Biopolymers, 1972, 11(10):2141-2145. http://d.old.wanfangdata.com.cn/Periodical/ccsfxyxb-z200704039 [9] HAYWARD J S, SMITH P J, KONDRAT S A, BOWKER M, HUTCHINGS G J. The effects of secondary oxides on copper-based catalysts for green methanol synthesis[J]. ChemCatChem, 2017, 9(9):1655-1662. doi: 10.1002/cctc.201601692 [10] WITOON T, CHALORNGTHAM J, DOMRONGBUNDITKUL P, CHAREONPANICH M, LIMTRAKUL J. CO2, hydrogenation to methanol over Cu/ZrO2, catalysts:Effects of zirconia phases[J]. Chem Eng J, 2016, 293:327-336. http://www.sciencedirect.com/science/article/pii/S138589471630170X [11] ZHANG H, LI F, GAO P, ZHAO N, XIAO F K, WEI W, ZHONG L S, SUN Y H. Methanol synthesis from CO2, hydrogenation over La-M-Cu-Zn-O (M=Y, Ce, Mg, Zr) catalysts derived from perovskite-type precursors[J]. J Power Sources, 2014, 251(251):113-121. https://www.sciencedirect.com/science/article/abs/pii/S0378775313018648 [12] BAN H, LI C, ASAMI K, FUJIMOTO K. Influence of rare-earth elements (La, Ce, Nd and Pr) on the performance of Cu/Zn/Zr catalyst for CH3OH synthesis from CO2[J]. Catal Commun, 2014, 54:50-54. https://www.sciencedirect.com/science/article/pii/S1566736714001952 [13] WITOON T, NUMPILAI T, PHONGAMWONG T, DONPHAI W, BOONYUEN CWARAKULWIT C, CHAREONPANICH M, LIMTRAKUL J. Enhancedactivity, selectivity and stability of a CuO-ZnO-ZrO2, catalyst by adding graphene oxide for CO2, hydrogenation to methanol[J]. Chem Eng J, 2018, 334:1781-1791. https://www.sciencedirect.com/science/article/pii/S1385894717320399 [14] DEERATTRAKUL V, DITTANET P, SAWANGPHRUK M, KONGKACHUICHAY P. CO2, hydrogenation to methanol using Cu-Zn catalyst supportedon reduced graphene oxide nanosheets[J]. J CO2 Util, 2016, 16:104-113. https://www.sciencedirect.com/science/article/pii/S221298201630155X [15] 程鹏泽, 高文桂, 纳薇, 王禹皓, 李艳艳, 徐毛毛.不同沉淀剂对CO2加氢合成甲醇Cu-ZnO-ZrO2催化剂性能的影响[J].化工进展, 2017, 36(8):2955-2961. http://d.old.wanfangdata.com.cn/Periodical/hgjz201708029CHENG Peng-ze, GAO Wen-gui, NA Wei, WANG Yu-hao, LI Yan-yan, XU Mao-mao. Effect of different precipitants on the performance of Cu-ZnO-ZrO2 catalyst for hydrogenation of CO2 to methanol[J]. Chem Ind Eng Prog, 2017, 36(8):2955-2961. http://d.old.wanfangdata.com.cn/Periodical/hgjz201708029 [16] PHONGAMWONG T, CHANTAPRASERTPORN U, WITOON T, NUMPILAI T CO2 hydrogenation to methanol over CuO-ZnO-ZrO2-SiO2 catalysts:Effects of SiO2contents[J]. Chem Eng J, 2017, 316:692-703. [17] 陈俊军, 高文桂, 王华, 纳薇. CaO对Cu-ZnO-ZrO2催化CO2加H2合成甲醇性能影响[J].燃料化学学报, 2016, 44(4):437-448. doi: 10.3969/j.issn.0253-2409.2016.04.008CHEN Jun-jun, GAO Wen-gui, WANG Hua, NA Wei. Effect of CaO on the Performance of Cu-ZnO-ZrO2 Catalyst for CO2 and H2 Synthesis of Methanol[J]. J Fuel Chem Technol, 2016, 44(4):437-448. doi: 10.3969/j.issn.0253-2409.2016.04.008 [18] DUMRONGBUNDITKUL P, WITOON T, CHAREONPANICH M, THUMRONGRUT M. Preparation and characterization of Co-Cu-ZrO2 nanomaterials and their catalytic activity in CO2 methanation[J]. Ceram Int, 2016, 42(8):10444-10451. doi: 10.1016/j.ceramint.2016.03.193 [19] LI Y Y, NA W, WANG H, GAO W G. Hydrogenation of CO2, to methanol over Au-CuO/SBA-15 catalysts[J]. J Porous Mater, 2016, 24(3):1-9. https://www.onacademic.com/detail/journal_1000039648292010_fe10.html [20] PEREZHERNANDEZ R, GUTIERREZMARTINEZ A, PALACIOS J, VEGAHERNANDEZ M, RODRIGUEZLUGO V. Hydrogen production by oxidativesteam reforming of methanol over Ni/CeO-ZrO catalysts[J]. Int J of Hydrogen Energy, 2011, 36(11):6601-6608. https://www.sciencedirect.com/science/article/pii/S0360319911004186 [21] ATAKAN A, MÄKIE P, SÖDERLIND F, KERAUDY J, BJORK E M, ODEN M. Synthesis of a Cu-infiltrated Zr-doped SBA-15 catalyst for CO2 hydrogenation into methanol and dimethyl ether.[J]. Phys Chem Chem Phys, 2017, 19(29):19139-19149. doi: 10.1039/C7CP03037A [22] WANG Y H, GAO W G, WANG H, ZHENG Y E, LI K Z, MA R G. Morphology and activity relationships of macroporous CuO-ZnO-ZrO2 catalysts for methanol synthesis from CO2 hydrogenation[J]. Rare Metals, 2016, 35(10):790-796. http://d.old.wanfangdata.com.cn/Periodical/xyjs-e201610009 [23] WANG F, WEI M, EVANS D G, DUAN X. CeO2-based heterogeneous catalysts toward catalytic conversion of CO2[J]. J Mater Chem A, 2016, 4:5773-5783. doi: 10.1039/C5TA10737G [24] ZHOU G L, DAI B C, XIE H M, ZHANG G Z, XIONG K, ZHENG X X. Ce-Cu composite catalyst for CO synthesis by reverse water-gas shift reaction:Effect of Ce/Cu mole ratio[J]. J CO2 Util, 2017, 21:292-301. https://www.sciencedirect.com/science/article/pii/S2212982017302111 [25] DAI B, ZHOU G, GE S, XIE H, JIAO Z, ZHANG G, XIONG K. CO2 reverse water-gas shift reaction on mesoporous M-CeO2 catalysts[J]. Can J Chem Eng, 2017, 95(4). doi: 10.1002/cjce.22730 [26] HONG L, HOU Z, XIE J. Hydrogenation of CO2 to CH3OH over CuO/ZnO/Al2O3 catalysts prepared via a solvent-free routine[J]. Fuel, 2016, 164:191-198. https://www.sciencedirect.com/science/article/pii/S0016236115009795 [27] GAO P, LI F, XIAO F K, ZGAO N, WEI W, ZHONG L S, SUN Y H. Effect of hydrotalcite-containing precursors on the performance of Cu/Zn/Al/Zr catalysts for CO2 hydrogenation:Introduction of Cu2+ at different formation stages of precursors[J]. Catal Today, 2012, 194(1):9-15. https://www.sciencedirect.com/science/article/pii/S0920586112004543 [28] DAI W L, SUN Q, DENG J F. XPS studies of Cu/ZnO/Al2O3, ultra-fine catalysts derived by a novel gel oxalate co-precipitation for methanol synthesis by CO2 +H2[J]. Appl Surf Sci, 2001, 177(3):172-179. https://www.sciencedirect.com/science/article/pii/S016943320100229X [29] LI C H, LI K Z, WANG H, ZHU X, WEI Y G, YAN D X, CHENG X M, ZHAI K. Soot combustion over Ce1-xFexO2-δ and CeO2/Fe2O3 catalysts:Roles of solid solution and interfacial interactions in the mixed oxides[J]. Appl Surf Sci, 2016, 390:513-525. doi: 10.1016/j.apsusc.2016.08.122 [30] ZENG L P, LI K Z, WANG H, YU H, ZHU X, WEI Y GNING P H, SHI C Z, LUO Y M. CO oxidation on Au/α-Fe2O3-hollow catalysts:General synthesis and structural dependence[J]. J Phys Chem C, 2017, 121(23). https://www.researchgate.net/publication/317129926_CO_Oxidation_on_Aua-Fe2O3-Hollow_Catalysts_General_Synthesis_and_Structural_Dependence [31] LI D Y, LI K Z, XU R D, WANG H, TIAN D, WEI Y G, ZHU X, ZENG C H, ZENG L P. Ce1-xFexO2-δ catalysts for catalytic methane combustion:Role of oxygen vacancy and structural dependence[J]. Catal Today, 2018, 318:73-85. [32] GAO P, YANG H, ZHANG L. Fluorinated Cu/Zn/Al/Zr hydrotalcites derived nanocatalysts for CO2, hydrogenation to methanol[J]. J CO2 Util, 2016, 16:32-41. doi: 10.1016/j.jcou.2016.06.001 [33] LEI H, NIE R F, WU G Q, HOU Z Y. Hydrogenation of CO2, to CH3OH over Cu/ZnO catalysts with different ZnO morphology[J]. Fuel, 2015, 154:161-166. doi: 10.1016/j.fuel.2015.03.052 [34] LIU Y X, SUN K P, MA H W, XU X L, XIAO L. Cr, Zr-incorporated hydrotalcites and their application in the synthesis of isophorone[J]. Catal Commun, 2010, 11(10):880-883. https://www.sciencedirect.com/science/article/pii/S1566736710000919 [35] WU G D, WANG X L, WEI W, SUN Y H. Fluorine-modified Mg-Al mixed oxides:A solid base with variable basic sites and tunable basicity[J]. Appl Catal A:Gen, 2010, 377(1):107-113. [36] GUO X M, MAO D S, LU G Z, WANG S, WU G S. The influence of La doping on the catalytic behavior of Cu/ZrO2 for methanol synthesis from CO2 hydrogenation[J]. J Mol Catal A:Chem, 2011, 345(1):60-68. https://www.researchgate.net/publication/251672048_The_influence_of_La_doping_on_the_catalytic_behavior_of_CuZrO_2_for_methanol_synthesis_from_CO_2_hydrogenation -





 下载:
下载: