-
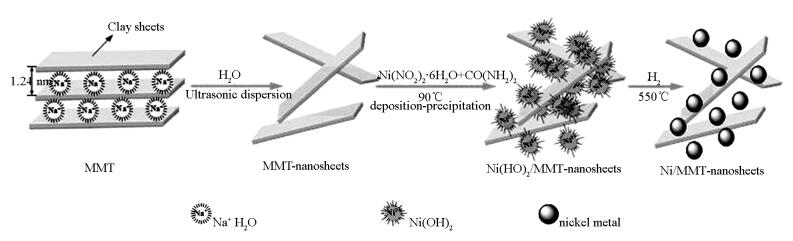
摘要: 以天然层状黏土蒙脱石(MMT)为前体,通过液相沉积-沉淀将镍物种引入水溶液中剥离为MMT纳米片表面的简易方法制得Ni/MMT纳米片。该Ni/MMT纳米片由于是二维(2D)结构,利于芳烃及其加氢产物的传质扩散,相比Ni/SBA-15和Ni/γ-Al2O3催化剂,具有更为高效的芳烃加氢性能,且在镍负载量高达18.5%时,其四氢萘加氢的转化频率(TOF)达到最高值。Abstract: Using montmorillonite (MMT), a natural layered clay, as the layered precursor, nanosheets of Ni/MMT are obtained via a facile method, in which the nickel components are introduced on to the surface of the exfoliated MMT nanosheets dispersed in water via deposition-precipitation with nickel nitrate and urea. Due to their unique properties originated from the two-dimensional (2D) structure, which favors mass transfer and diffusion of the aromatics and their hydrogenation products over the catalyst during reaction, the obtained nanosheets of Ni/Clay show higher efficient for hydrogenation of aromatics than Ni/SBA-15 and Ni/γ-Al2O3 catalysts. And the highest TOF for hydrogenation of tetralin over the nanosheets of Ni/Clay is obtained as nickel loading being high to 18.5%.
-
Key words:
- nanaosheets /
- Ni/Clay /
- montmorillonite /
- hydrogenation /
- aromatics
-
Table 1 Textural properties of the catalysts determined by N2 adsorption
Catalyst Ni Loading
w/%BET surface
area A/(m2·g-1)Pore volume
v/(cm3·g-1)Pore diameter
d/nmMMT - 9 0.05 20.9 Ni/MMT 9.9 9 0.04 15.5 Ni/MMT-ultrasonic 10.1 44 0.10 8.9 Ni/MMT-nanosheets 12.3 144 0.23 6.5 Ni/SBA-15[20] 10.2 334 0.69 7.5 Table 2 Activity of Ni/MMT-nanosheet for hydrogenation of naphthalene a
Catalyst Ni loading
w/%Conversion
x/%Selectivity s/% TOFb/
h-1Ni0 size
d/nmtetralin decalin Ni/MMT 9.9 13.1 99.3 0.7 5.1 19.8 Ni/MMT-ultrasonic 10.1 19.8 99.1 0.9 7.6 - Ni/MMT-nanosheet 12.3 100.0 85.4 14.6 37.8 7.2 Ni/SBA-15 [20] 10.2 68.2 93.6 6.4 28.0 3.7 Ni/γ-Al2O3 [20], c 9.8 42.5 99.2 0.8 8.4 2.2 a: reaction conditions: the solution of naphthalene in n-dodecane (10.0%) 10 g, catalyst 0.12 g, 300 ℃, p(H2) = 5.0 MPa, 2.0 h; b: turnover frequency (TOF) was defined as number of moles of consumed H2 per mole of Ni per hour; c: solution of naphthalene in n-dodecane is 5.0% for reaction Table 3 Activities of Ni/MMT-nanosheet with different nickel loading for hydrogenation of tetralina
Catalyst Ni loading
w/%Conversion
x/%TOFb/
h-1Ni/MMT 9.9 2.1 8.5 Ni/MMT-ultrasonic 10.1 3.7 14.6 5.7 11.7 82.0 Ni/MMT-nanosheets 12.3 35.0 113.7 18.5 78.6 169.8 27.7 55.2 79.6 a: reaction conditions: tetralin 3.0 g, catalyst 0.05 g, 300 ℃, p(H2) = 5.0 MPa, 2.0 h; b: turnover frequency (TOF) was defined as number of moles of consumed H2 per mole of Ni per hour Table 4 Physical properties of Ni/MMT-nanosheet catalysts with different nickel loadings
Catalyst Ni w/% BET surface area A/(m2·g-1) Pore volume v/(cm3·g-1) Pore diameter d/nm Ni/MMT-nanosheet 5.7 128 0.22 6.6 12.3 144 0.23 6.5 18.5 168 0.38 9.1 27.7 125 0.37 11.9 -
[1] CHOI M, NA K, KIM J, SAKAMOTOY, TERASAKI O, RAYOO R. Stable single-unit-cell nanosheets of zeolite MFI as active and long-lived catalysts[J]. Nature, 2009, 461:246-249. doi: 10.1038/nature08288 [2] ROTH W J, NACHTIGALL P, MORRIS R E, ČEJKA J. Two-Dimensional zeolites:Current status and perspectives[J]. Chem Rev, 2014, 114(9):4807-4837. doi: 10.1021/cr400600f [3] OGINO I, NIGRA M M, HWANG S J, HA J M, REA T, STACEY I, ZONES S I, KATZ A. Delamination of layered zeolite precursors under mild conditions:Synthesis of UCB-1 via fluoride/chloride anion-promoted exfoliation[J]. J Am Chem Soc, 2011, 1339(10):3288-3291. https://core.ac.uk/download/pdf/4888769.pdf [4] EILERTSEN E A, OGINO I, HWANG S J, REA T, YEH S, ZONES S I, KATZ A. Nonaqueous fluoride/chloride anion-promoted delamination of layered zeolite precursors:Synthesis and characterization of UCB-2[J]. Chem Mater, 2011, 23(24):5404-5408. doi: 10.1021/cm202364q [5] AN Z, HE J, DUAN X. Catalysts with catalytic sites highly dispersed from layered double hydroxide as precursors[J]. Chin J Catal, 2013, 34:225-234. https://www.researchgate.net/publication/275884411_Catalysts_with_catalytic_sites_highly_dispersed_from_layered_double_hydroxide_as_precursors [6] WANG J, ZHAO L, SHI H, HE J. Highly enantioselective and efficient asymmetric epoxidation catalysts:Inorganic nanosheets modified withα-amino acids as ligands[J]. Angew Chem Int Ed, 2011, 50:9171-9176. doi: 10.1002/anie.201103713 [7] YAO H B, MAO L B, YAN Y X, CONG H P, LEI X, YU S H. Gold nanoparticle functionalized artificial nacre:Facile in situ growth of nanoparticles on montmorillonite nanosheets, self-assembly, and their multiple properties[J]. ACS Nano, 2012, 6(9):8250-8260. doi: 10.1021/nn3029315 [8] YAO H B, TAN Z H, FANG H Y, YU S H. Artificial nacre-like bionano-composite films from the self-assembly of chitosan-montmor-illonite hybrid building blocks[J]. Angew Chem Int Ed, 2010, 49:10127-10131. doi: 10.1002/anie.201004748 [9] INTROZZI L, BLOMFEID O J T, TRABATTONI S, TAVAZZI S, SANTO N, SCHIRALDI A, PIERGIOVANNI L, FARRIS S. Ultrasound-assisted pullulan/montmorillonite bionanocomposite coating with high oxygen barrier properties[J]. Langmuir, 2012, 28(30):11206-11214. doi: 10.1021/la301781n [10] GIL A, KORILI S A, VICENTE M A. Recent advances in the synthesis and catalytic application of pillared clay catalysts[J]. Catal Rev Sci Eng, 2008, 50:153-221. doi: 10.1080/01614940802019383 [11] ZHANG W J, LI K S M, WANG R J, YUE P L, Gao P. Preparation of stable exfoliated Pt-Clay nanocatalyst[J]. Langmuir, 2009, 25(14):8226-8234. doi: 10.1021/la900416v@proofing [12] ONG W J, PUTRI L K, TAN L L, CHAI S P, YONG S T. Heterostructured AgX/g-C3N4 (X=Cl and Br) nanocomposites via a sonication-assisted deposition-precipitation approach:Emerging role of halide ions in the synergistic photocatalytic reduction of carbon dioxide[J]. Appl Catal B:Environ, 2016, 180:530-543. doi: 10.1016/j.apcatb.2015.06.053 [13] SKAF M, AOUAD S, HANY S, COUSIN R, ABI-AAD E, ABOUKAIS A. Physicochemical characterization and catalytic performance of 10% Ag/CeO2 catalysts prepared by impregnation and deposition-precipitation[J]. J Catal, 2014, 320:137-146. doi: 10.1016/j.jcat.2014.10.006 [14] KITTISAKMONTREE P, PONGTHAWORNSAKUN B, YOUSHIDA H, FUJITA S, ARAI M, PANPRANOT J. The liquid-phase hydrogenation of 1-heptyne over Pd-Au/TiO2 catalysts prepared by the combination of incipient wetness impregnation and deposition-precipitation[J]. J Catal, 2003, 297:155-164. https://file.scirp.org/Html/2-2530027_29896.htm [15] BURATTIN B, CHE M, LOUIS C. Ni/SiO2 materials prepared by deposition-precipitation:Influence of the reduction conditions and mechanism of formation of metal particles[J]. J Phys Chem B, 2000, 104(45):10482-10489. doi: 10.1021/jp0003151 [16] LIU H C, WANG H, SHEN J H, SUN Y, LIU Z M. Preparation, characterization and activities of the nano-sized Ni/SBA-15 catalyst for producing COx -free hydrogen from ammonia[J]. Appl Catal A:Gen, 2008, 337(2):138-147. doi: 10.1016/j.apcata.2007.12.006 [17] GOMEZ-REYNOSO R, RAMLREZ J, NARES R, LUNA R, MURRIETA F. Characterization and catalytic activity of Ni/SBA-15, synthesized by deposition-precipitation[J]. Catal Today, 2005, 107/108:926-932. doi: 10.1016/j.cattod.2005.07.152 [18] KIM H J, SONG C S. Enhancing sulfur tolerance of Pd catalysts by hydrogen spillover with two different zeolite supports for low-temperature hydrogenation of aromatics[J]. Energy Fuels, 2014, 28(11):6788-6792. doi: 10.1021/ef501541j [19] KIRUMAKKI S R, SHPEIZER B G, SAGAR G V, CHARY K V R, CLEARFIELD A. Hydrogenation of naphthalene over NiO/SiO2-Al2O3 catalysts:Structure-activity correlation[J]. J Catal, 2006, 242(2):319-331. doi: 10.1016/j.jcat.2006.06.014 [20] REN S B, ZHAO R, ZHANG P, LEI Z P, WANG Z C, KANG S G, PAN C X, SHUI H F. Effect of activation atmosphere on the reduction behaviors, dispersion and activities of nickel catalysts for the hydrogenation of naphthalene[J]. Reac Kinet Mech Cat, 2014, 111(1):247-257. doi: 10.1007/s11144-013-0629-3 -





 下载:
下载: