Modified solid superacid S2O82-/ZrO2-CoO for oxidative desulfurization of FCC gasoline
-
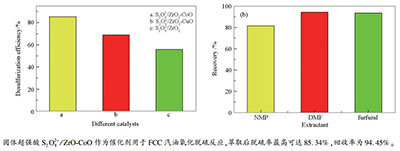
摘要: 以硝酸锆、硝酸铜和硝酸钴为金属源,过硫酸铵作为浸渍液,采用共沉淀浸渍法合成出固体超强酸催化剂S2O82-/ZrO2、S2O82-/ZrO2-CuO和S2O82-/ZrO2-CoO,通过XRD、FT-IR、NH3-TPD、BET对催化剂进行表征。结果表明,Co(钴)改性催化剂S2O82-/ZrO2-CoO在三种催化剂中超强酸位最多。将其作为催化剂,过氧化氢作为氧化剂用于FCC汽油氧化脱硫反应,研究不同反应温度、催化剂用量、反应时间、氧化剂用量对FCC汽油脱硫效果的影响。结果表明,FCC汽油氧化脱硫的最佳条件为:反应温度70 ℃,反应1.5 h,FCC汽油加入量与氧化剂体积比7.5:1,催化剂用量0.02 g/mL。反应产物利用N,N-二甲基甲酰胺进行萃取分离,萃取剂/汽油体积比为1:1时,FCC汽油脱硫率最高可达85.34%,回收率为94.45%,并且催化剂表现出较为稳定的催化活性。Abstract: A series of solid super acid catalysts, S2O82-/ZrO2, S2O82-/ZrO2-CuO and S2O82-/ZrO2-CoO, were synthesized by coprecipitation impregnation method with zirconium nitrate, copper nitrate and cobalt nitrate as the metal sources and with ammonium persulfate as the impregnation solution. The catalysts were characterized by XRD, FT-IR and NH3-TPD. The characterization shows that the S2O82-/ZrO2-CoO catalyst has the most super acid sites among the three catalysts. S2O82-/ZrO2-CoO as the catalyst and hydrogen peroxide as the oxidant were used for oxidative desulfurization of FCC gasoline, and the effects of reaction temperature, catalyst dosage, reaction time and oxidant dosage on the desulfurization of FCC gasoline were studied. The optimal conditions were determined as:FCC gasoline of 15 mL, reaction temperature of 70℃, reaction time of 1.5 h, oxidant dosage of V(H2O2):V(oil)=7.5:1, and catalyst dosage of 0.02 g/mL. Moreover, the reaction product was extracted with N, N-dimethylformamide. When the volume ratio of extractant to gasoline is 1:1, the sulfur removal efficiency and recovery of FCC gasoline reach to 85.34% and 94.45%, respectively. The catalyst exhibits a relatively stable catalytic activity.
-
Key words:
- solid super acid /
- oxidative desulfurization /
- impregnation method /
- FCC gasoline
-
表 1 不同催化剂的BET数据
Table 1 BET data of different catalysts
Catalyst Surface area A/(m2·g-1) Pore volume v/(cm3·g-1) Pore diameter d/nm S2O82-/ZrO2 102.2 0.129 1.83 S2O82-/ZrO2-CuO 114.1 0.098 0.96 S2O82-/ZrO2-CoO 130.9 0.071 0.55 表 2 直接萃取与反应后再萃取对脱硫率的影响
Table 2 Effect of direct extraction and extraction after reaction on the desulfurization efficiency
Extraction Desulfurization efficiency η/% Recovery /% Direct extraction 49.85 95.33 Extraction after reaction 85.34 94.45 表 3 FCC汽油氧化脱硫反应前后的硫形态分布
Table 3 Distribution of sulfur form before and after oxidative desulfurization of FCC gasoline
Apex RT Species of sulfide Distribution w/% sbefore safter 1.27 hydrothion 35.32 - 1.33 methanthiol 36.19 8.92 1.47 ethanethiol 136.83 33.93 1.65 isopropyl mercaptan 32.89 8.14 1.94 n-propyl mercaptan 50.37 12.60 2.48 thiophene 166.31 41.30 3.14 n-butyl mercaptan 10.83 2.71 4.62 2-methylthiophene 94.12 23.46 4.84 3-methylthiophene 125.76 - 5.69 tetrahydrothiophene 57.05 - 6.93 C6-thioether 15.12 3.68 7.51 2-methyltetrahydrothiophene 15.89 - 8.69 2-ethylthiophene 61.67 - 9.35 2、4-dimethylthiophene 77.92 19.2 9.83 2、3-dimethylthiophene 55.92 - 10.64 3、5-dimethylthiophene 31.86 - 15.23 C3-thiophene 34.18 - 15.4 C3-thiophene 12.50 - 15.86 C3-thiophene 36.53 9.11 17.05 C3-thiophene 8.47 - 17.84 C3-thiophene 31.61 - 21.88 C4-thiophene 19.31 4.85 23.65 C4-thiophene 6.54 - 24.51 C4-thiophene 6.76 - 25.38 C4-thiophene 9.22 - 30.06 benzothiophene 76.84 19.00 34.26 C4-thiophene 6.80 - 36.38 methylbenzothiophene 11.6 - 36.95 methylbenzothiophene 11.05 - 37.44 methylbenzothiophene 8.17 - 37.8 methylbenzothiophene 12.32 3.10 Total 1295.96 190.02 -
[1] TAYLOR H J, BELL J N B. Studies on the tolerance to SO2 of grass populations in polluted areas[J]. New Phytol, 1988, 110(3):327-338. doi: 10.1111/nph.1988.110.issue-3 [2] LAM V, LI G, SONG C, CHEN J, FAIRBRIDGE C, HUI R, ZHANG J. A review of electrochemical desulfurization technologies for fossil fuels[J]. Fuel Process Technol, 2012, 98(0):30-38. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=b08cad4cb0d1dac649df5717d2123676 [3] STANISLAUS A, MARAFI A, RANA M S. Recent advances in the science and technology of ultra low sulfur diesel (ULSD) production[J]. Catal Today, 2010, 153(1/2):1-68. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=c3a5d46f7b195464cfb15c24c522231a [4] 蒋国珍.加工高硫原油设备腐蚀与防护对策[J].机械制造与自动化, 2003, 32(2):38-42. doi: 10.3969/j.issn.1671-5276.2003.02.013JIANG Guo-zhen. Strategies of equipment erosion protection in refinery processing high sulfur crude oil[J]. Mach Build Autom, 2003, 32(2):38-42. doi: 10.3969/j.issn.1671-5276.2003.02.013 [5] 李永安.炼油生产过程硫的分布追踪与平衡[J].石油化工腐蚀与防腐, 2001, 18(4):3-8. http://d.old.wanfangdata.com.cn/Periodical/syhgfsyfh200104012LI Yong-an. Trace and balance of sulfur distribution in refinery production process[J]. Petrochem Corros Protect, 2001, 18(4):3-8. http://d.old.wanfangdata.com.cn/Periodical/syhgfsyfh200104012 [6] BRUNET S, MEY D, PÉROT G, BOUCHY C, DIEHL F. On the hydrodesulfurization of FCC gasoline:A review[J]. Appl Catal A:Gen, 2005, 278(2):143-172. doi: 10.1016/j.apcata.2004.10.012 [7] 聂毅, 李春喜, 孟洪, 王子镐.汽柴油深度脱硫的技术研究进展[J].当代化工, 2006, 35(6):409-413. doi: 10.3969/j.issn.1671-0460.2006.06.016NIE Yi, LI Chun-xi, MENG Hong, WANG Zi-hao. Research progress on deep desulfurization technology of gasoline and diesel[J]. Contemp Chem Ind, 2006, 35(6):409-413. doi: 10.3969/j.issn.1671-0460.2006.06.016 [8] NAPANANG T, SOOKNOI T. Oxidative extraction of thiophene fromn-dodecane over TS-1 in continuous process:A model for non-severe sulfur removal from liquid fuels[J]. Catal Commun, 2010, 11(1):1-6. http://www.sciencedirect.com/science/article/pii/S1566736709002921 [9] ZHAO H, BACKER G A, ZHANG Q. Design rules of ionic liquids tasked for highly efficient fuel desulfurization by mild oxidative extraction[J]. Fuel, 2017, 189:334-339. doi: 10.1016/j.fuel.2016.10.109 [10] MOGHADAM F R, AZIZIAN S, KIANPOUR E, YARIE M, BAYAT M, ZOLFIGOL M A. Green fuel through green route by using task-specific and neutral phosphonium ionic liquid:A joint experimental and theoretical study[J]. Cat Sci Eng, 2017, 309:480-488. http://www.sciencedirect.com/science/article/pii/S1385894716314279 [11] ECORMIERM A, WILSON K, LEE A F. Structure reactivity correlations in sulphated-zirconia catalysts for the isomerisation of α-pinene[J]. J Catal, 2003, 215(1):57-65. doi: 10.1016/S0021-9517(02)00150-1 [12] KIMURA T. Development of Pt/SO42-/ZrO2 catalyst for isomerization of light naphtha[J]. Catal Today, 2003, 81(1):57-63. doi: 10.1016/S0920-5861(03)00102-0 [13] SHAH A K, KUMAR M, ABDI S H R, KURESHY R I, KHAN N H, BAJAJ H C. Solvent-free aminolysis of aliphatic and aryloxy epoxides with sulfated zirconia as solid acid catalyst[J]. Appl Catal A:Gen, 2014, 486:105-114. doi: 10.1016/j.apcata.2014.08.024 [14] ZHANG C, ZHANG J, ZHAO Y, SUN J, WU G. Study on the preparation and catalytic activities of SO42- promoted metal oxide solid superacid catalysts for model oil desulfurization[J]. Catal Lett, 2016, 146(7):1256-1263. doi: 10.1007/s10562-016-1744-3 [15] 夏勇德, 华伟明, 高滋. S2O82-处理的ZrO2固体超强酸上的正丁烷异构化反应[J].化学学报, 1999, 57(12):1325-1331. doi: 10.3321/j.issn:0567-7351.1999.12.007XIA Yong-de, HUA Wei-ming, GAO Zi. Isobutane isomerization of ZrO2 solid superacid treated by S2O82-[J]. Acta Chim Sin, 1999, 57(12):1325-1331. doi: 10.3321/j.issn:0567-7351.1999.12.007 [16] SONG H, WANG N, SONG H-L, LI F. La-Ni modified S2O82-/ZrO2-Al2O3 catalyst in n-pentane hydroisomerization[J]. Catal Commun, 2015, 59(2015):61-64. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=e4c0b849bb76c6da6b73c59c70b90651 [17] LIU Z X, NIE X A, WANG Y G. Biodiesel preparation from styrax confusus hemsl oil catalyzed by magnetic catalyst S2O82-/ZrO2-Fe3O4[J]. Adv Mater Res, 2013, 805-806:247-250. doi: 10.4028/www.scientific.net/AMR.805-806 [18] SHEN M. Synthesis of butyl lactate on S2O82-/ZrO2-CeO2 solid superacid catalyst[J]. Appl Chem Ind, 2011, 40(5):853-855. [19] 姚元勇, 唐帮成, 陈仕学, 邢明明, 吴思展, 舒华. S2O82-/TiO2或SO42-/TiO2固体超强酸催化甲氧基杨梅苷水解脱苷研究[J].食品科技, 2015, 40(7):281-285. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=spkj201507057YAO Yuan-yong, TANG Bang-cheng, CHEN Shi-xue, XING Ming-ming, WU Si-zhan, SHU Hua. Catalytic hydrolysis of methoxy myricetin by applying solid super acids S2O82-/TiO2or SO42-/TiO2[J]. Food Sci Technol, 2015, 40(7):281-285. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=spkj201507057 [20] 郭宁, 杨冲, 刘振学, 候影飞, 王然, 李春虎.活性炭负载S2O42-/ZrO2催化剂的制备及其催化氧化脱硫性能[J].石油学报(石油加工), 2015, 31(6):1416-1424. doi: 10.3969/j.issn.1001-8719.2015.06.024GUO Ning, YANG Chong, LIU Zhen-xue, HOU Ying-fei, WANG Ran, LI Chun-hu. Preparation and performance of activated carbon loaded S2O42-/ZrO2 catalyst for catalytic oxidative desulfurization[J]. Acta Pet Sin(Pet Process Sect), 2015, 31(6):1416-1424. doi: 10.3969/j.issn.1001-8719.2015.06.024 [21] 罗瑞生.固体超强酸用于FCC汽油催化氧化脱硫的研究[D].青岛: 中国海洋大学, 2009. http://d.wanfangdata.com.cn/Thesis_Y1503604.aspxLUO Rui-sheng. Study on oxidative desulfurization of gasoline over super solid acid catalyst[D]. Qingdao: Ocean University of China, 2009. http://d.wanfangdata.com.cn/Thesis_Y1503604.aspx [22] 郭宁, 侯影飞, 吴明铂, 李春虎.多孔炭负载固体超强酸催化氧化脱硫工艺研究[J].长春工程学院学报:自然科学版, 2013, 14(2):112-114.) http://d.old.wanfangdata.com.cn/Periodical/ccgcxyxb201302032GUO Ning, HOU Ying-fei, WU Ming-bo, LI Chun-hu. Research on catalyst oxidation and desulfurization process to porous carbon loaded solid super-acid[J]. J Changchun Inst Technol (Nat Sci Ed), 2013, 14(2):112-114. http://d.old.wanfangdata.com.cn/Periodical/ccgcxyxb201302032 [23] 曹小华, 占昌朝, 任杰, 谢宝华, 黄星星, 陶春元. S2O42-/ZrO2-WO3复合固体超强酸的制备和表征及其催化合成环己烯的性能[J].石油化工, 2010, 39(1):36-41. http://www.cqvip.com/Main/Detail.aspx?id=32741733CAO Xiao-hua, ZHAN Chang-chao, REN Jie, XIE Bao-hua, HUANG Xing-xing, TAO Chun-yuan. Preparation and characterization of composite solid super acid and its catalytic activity in synthesis of cyclohexene[J]. Petrochem Technol, 2010, 39(1):36-41. http://www.cqvip.com/Main/Detail.aspx?id=32741733 [24] MARCAEWSKI M, KAMINSKAE, MARCZEWSKA H. Decomposition of styrene dimers:The influence of the acid strength of the catalyst[J]. React Kinet Mech Catal, 2013, 108(1):59-68. doi: 10.1007/s11144-012-0496-3 [25] YANG F, LI Y, ZHANG Q, SUN X, FAN H, XU N, LI G. Selective conversion of cotton cellulose to glucose and 5-hydroxymethyl furfural with SO42-/MxOy solid superacid catalyst[J]. Carbohydr Polym, 2015, 131:9-14. doi: 10.1016/j.carbpol.2015.05.036 [26] LEE J S, PARK D S. Interaction of pyridine and ammonia with a sulfate-promoted iron oxide catalyst[J]. J Catal, 1998, 120(1):46-54. http://www.sciencedirect.com/science/article/pii/0021951789902492 [27] OTSUKI S, NONAKA T, TAKASHIMA N, QIAN W, ISHIHARA A, IMAI T, KABE T. Oxidative desulfurization of light gas oil and vacuum gas oil by oxidation and solvent extraction[J]. Energy Fuels, 2000, 14(6):1232-1239. doi: 10.1021/ef000096i [28] 单玉华, 邬国英, 李为民, 黄卫.杂多酸催化下过氧化氢氧化法精制焦化汽油[J].石油化工, 2003, 32(5):361-364. doi: 10.3321/j.issn:1000-8144.2003.05.001SHAN Yu-hua, WU Guo-ying, LI Wei-min, HUANG Wei. A new oxidation process for coking gasoline refining using hydrogenperoxide as oxidant[J]. Petrochem Technol, 2003, 32(5):361-364. doi: 10.3321/j.issn:1000-8144.2003.05.001 [29] SH/T 0689-2000, 轻质烃及发动机燃料和其他油品的总硫含量测定法(紫外荧光法)[S].SH/T 0689-2000, Standard test method for determination of total sulfur in light hydrocarbons motor fuels and oils by ultraviolet fluorescence[S]. [30] MIAO C, HUA W, CHEN J, GAO Z. Studies on SO42- promoted mixed oxide superacids[J]. Catal Lett, 1996, 37(3/4):187-191. [31] WANG H G, SHI G L, YU F, LI R F. Mild synthesis of biofuel over a microcrystalline S2O42-/ZrO2 catalyst[J]. Fuel Process Technol, 2016, 145:9-13. doi: 10.1016/j.fuproc.2016.01.021 [32] 曾飞虎, 王雪娥, 陈雪平. SiO2或TiO2改性S2O42-/ZrO2固体超强酸的结构及其催化性能[J].石油化工, 2013, 42(4):368-373. doi: 10.3969/j.issn.1000-8144.2013.04.002ZENG Fei-hu, WANG Xue-e, CHEN Xue-ping. Structure and catalystic performance of S2O82-/ZrO2 modified by SiO2 or TiO2[J]. Petrochem Technol, 2013, 42(4):368-373. doi: 10.3969/j.issn.1000-8144.2013.04.002 [33] SONG H, DONG P-H, LI F, JIN Z. Effect of al content on the isomerization performance of solid superacid Pd-S2O82-/ZrO2 -Al2O3[J]. Chem J Chin Univ, 2010, 22(11/12):1226-1231. [34] DESHMANE V G, ADEWUYI Y G. Mesoporous nanocrystalline sulfated zirconia synthesis and its application for FFA esterification in oils[J]. Appl Catal A:Gen, 2013, 462(27):196-206. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=914de2ce5f17908687af8574ab84e1f9 [35] 占华端, 陈晓晖, 魏可镁.固体超强酸催化剂S2O82-/Fe2O3-Al2O3的制备及其酯化性能[J].工业催化, 2010, 18(8):23-29. doi: 10.3969/j.issn.1008-1143.2010.08.005ZHAN Hua-duan, CHEN Xiao-hui, WEI Ke-mei. Preparation of S2O82-/Fe2O3-Al2O3 solid superacid and its behaviors for esterification[J]. Ind Catal, 2010, 18(8):23-29. doi: 10.3969/j.issn.1008-1143.2010.08.005 [36] 吴洪达, 郭敏. Keggin结构铜单取代硅钨酸钾催化过氧化氢分解[J].精细石油化工, 2003, 20(1):8-11. http://d.old.wanfangdata.com.cn/Periodical/jxsyhg200301003WU Hong-da, GUO Min. Keggin structure copper monosubstituted potassium tungstate potassium catalyzed decomposition of hydrogen peroxide[J]. Spec Petrochem, 2003, 20(1):8-11. http://d.old.wanfangdata.com.cn/Periodical/jxsyhg200301003 [37] 许志忠, 李晓春.过氧化氢分解影响因素分析[J].染整技术, 2006, 28(1):33-35+38. doi: 10.3969/j.issn.1005-9350.2006.01.011XU Zhi-zhong, LI Xiao-chun. Analysis of factors affecting hydrogen peroxide decomposition[J]. Text Dyeing Finish J, 2006, 28(1):33-35+38. doi: 10.3969/j.issn.1005-9350.2006.01.011 [38] GB/T 33318-2016, 气体分析硫化物的测定硫化学发光气相色谱法[S].GB/T 33318-2016, Gas analysis-determination of sulfide-gas chromatography with sulfur chemiluminescence[S]. -





 下载:
下载: