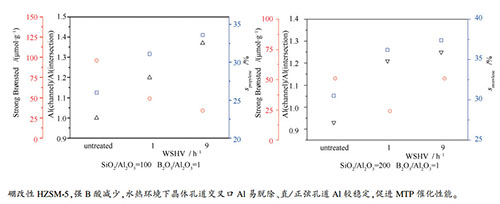
-
摘要: 水热晶化一步合成了BHZSM-5分子筛,投料SiO2/Al2O3=100、200,其中,B2O3/Al2O3=1,研究了其甲醇制丙烯(MTP)催化活性。硼改性提高了丙烯选择性,并有利于稳定活性。硼修饰引起合成样品的强B酸量减少;水热(480℃)环境条件下,BHZSM-5的强B酸量保留量约50%,相比HZSM-5,酸性位点保留较多,显示增强的水热稳定性;同时骨架Al分布发生了变化:位于晶体直孔道和正弦孔道的Al稳定,孔道交叉口的Al易于脱除,有利于基于烯烃循环机理的MTP活性。水热处理空速由1 h-1增加到9 h-1,B酸量进一步下降,晶体孔道交叉口的Al脱除更多。Abstract: BHZSM-5 zeolite was synthesized using one-step hydrothermal crystallization by changing SiO2/Al2O3 (100 and 200) with B2O3/Al2O3=1. The catalytic activity for methanol to propylene (MTP) was studied. Boron modification lead to increased propylene selectivity and improved stability. Boron modification reduced the amount of the strong Brønsted (B) acid sites. Subjected to hydrothermal treatment at 480 ℃, BHZSM-5 remained 50% of the amount of the strong B acid sites, higher than that of HZSM-5, showing enhanced hydrothermal stability. The distribution of the framework Al also changed. The Al located in the straight and the sinusoidal channels of the ZSM-5 crystal was stable whereas those at the channel intersections was easy to be removed, favoring the MTP activity via the olefin cycle mechanism. As the hydrothermal treatment velocity was increased from 1 h-1 to 9 h-1, the B acid sites amount of the BHZSM-5 further decreased and more Al at the intersections was removed.
-
Key words:
- boron modification /
- HZSM-5 /
- methanol to propylene /
- Brønsted acidity /
- Al distribution
-
表 1 样品命名及投料配比
Table 1 Names and charging compositions of synthetic samples
Name SiO2 /Al2O3 B2O3 /Al2O3 SiO2 /(Al2O3+B2O3) BHZ-5-100 100 1 50 HZ-5-100 100 0 100 BHZ-5-200 200 1 100 HZ-5-200 200 0 200 表 2 合成样品的Py-FTIR酸性
Table 2 Acidity of synthetic samples derived from Py-FTIR
Sample B /(μmol·g-1) Total B/(μmol·g-1) Total L /(μmol·g-1) weak medium strong strong BHZ-5-100 225 107 96 428 12 HZ-5-100 102 89 174 365 55 BHZ-5-200 178 136 51 365 10 HZ-5-200 123 56 94 273 41 表 3 样品的孔结构
Table 3 Textural properties of different samples
Sample WSHV/h-1 Crystallinity /% ABET/(m2·g-1) vtotal/(cm3·g-1) vmicro/(cm3·g-1) vmeso/(cm3·g-1) BHZ-5-100 1 108 352 0.206 0.137 0.069 HZ-5-100 1 163 359 0.231 0.141 0.090 BHZ-5-100 0 99 395 0.201 0.142 0.059 HZ-5-100 0 100 388 0.200 0.146 0.054 BHZ-5-100 9 106 349 0.199 0.136 0.063 HZ-5-100 9 166 347 0.214 0.135 0.079 表 4 样品的峰面积
Table 4 Proportions of deconvolution peak areas for different samples
Samples WSHV/h-1 Proportions /% Al(d)/Al(c) Al(e) 58 Al(d) 56 Al(c) 54 Al(b) 53 Al(a) 52 BHZ-5-100 1 12 30 25 17 15 1.20 HZ-5-100 1 10 25 23 19 21 1.08 BHZ-5-200 1 6 28 23 16 26 1.21 HZ-5-200 1 4 28 27 20 21 1.03 BHZ-5-100 0 4 29 29 18 19 1.00 HZ-5-100 0 4 29 23 18 25 1.26 BHZ-5-200 0 4 29 31 4 31 0.93 HZ-5-200 0 4 29 29 4 33 1.00 BHZ-5-100 9 13 31 23 16 16 1.37 HZ-5-100 9 9 26 24 20 19 1.08 BHZ-5-200 9 5 30 24 16 24 1.25 HZ-5-200 9 5 27 25 20 23 1.08 表 5 样品的Py-FTIR酸性
Table 5 Acidity of samples derived from Py-FTIR
Sample WSHV /h-1 B /(μmol·g-1) Total B /(μmol·g-1) Total L /(μmol·g-1) weak medium strong strong BHZ-5-100 1 120 22 49 191 2 HZ-5-100 1 93 31 40 164 38 BHZ-5-200 1 69 25 24 118 0 HZ-5-200 1 49 34 22 105 24 BHZ-5-100 9 122 36 34 192 2 HZ-5-100 9 53 42 31 126 29 BHZ-5-200 9 98 2 51 151 0 HZ-5-200 9 27 31 11 69 21 表 6 水热处理样品的MTP产物选择性
Table 6 Product selectivity of MTP on hydrothermally treated samples
Samples WSHV /h-1 Selectivity s/% C3=/C2= HTI C1 C2= C2 C3= C3 C4= C5+ BHZ-5-100 1 5.0 26.2 0.3 31.1 3.5 8.8 16.1 1.18 0.31 HZ-5-100 1 4.3 20.4 0.2 27.5 2.5 7.3 20.2 1.35 0.32 BHZ-5-200 1 2.6 26.9 0.2 36.2 2.3 10.4 14.5 1.35 0.20 HZ-5-200 1 2.8 23.9 0.2 36.3 2.2 9.6 17.2 1.52 0.22 BHZ-5-100 0 8.3 28.7 0.7 26.0 5.1 7.1 17.2 0.91 0.36 HZ-5-100 0 9.8 27.4 0.7 22.6 5.4 6.1 21.1 0.82 0.43 BHZ-5-200 0 6.2 28.3 0.4 30.5 3.7 7.7 17.5 1.08 0.25 HZ-5-200 0 7.8 28.4 0.6 27.7 5.1 7.7 17.0 0.97 0.31 BHZ-5-100 9 4.4 25.3 0.3 33.6 2.6 9.2 17.1 1.33 0.24 HZ-5-100 9 4.9 25.5 0.2 32.9 2.8 9.5 16.1 1.29 0.26 BHZ-5-200 9 2.3 27.6 0.3 37.4 3.3 9.5 11.2 1.35 0.25 HZ-5-200 9 1.9 23.8 0.3 37.4 3.1 10.0 15.8 1.52 0.23 time-on-stream: 6 h; methanol conversion: 100% -
[1] TARACH K A, MARTINEZ-TRIGUERO J, REY F, GÓRA-MAREK K. Hydrothermal stability and catalytic performance of desilicated highly siliceous zeolites ZSM-5[J]. J Catal, 2016, 339:256-269. doi: 10.1016/j.jcat.2016.04.023 [2] BENITO P L, GAYUBO A G, AGUAYO A T, OLAZAR M, BILBAO J. Deposition and characteristics of coke over a H-ZSM-5 zeolite-based catalyst in the MTG process[J]. Ind Eng Chem Res, 1996, 35:3991-3998. doi: 10.1021/ie950462z [3] 温鹏宇, 梅长松, 刘红星, 杨为民, 陈庆龄. ZSM-5硅铝比对甲醇制丙烯反应产物的影响[J].化学反应工程与工艺, 2007, 23(5):385-390. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hxfygcygy200705001WEN Peng-yu, MEI Chang-song, LIU Hong-xing, YANG Wei-min, CHEN Qing-ling. Effect of Si/Al ratio in ZSM-5 on the selectivity of products for methanol conversion to propylene[J]. Chem React Eng Technol, 2007, 23(5):385-390. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=hxfygcygy200705001 [4] ZHAO T S, TAKEMOTO T, TSUBAKI N. Direct synthesis of propylene and light olefins from dimethyl ether catalyzed by modified H-ZSM-5[J]. Catal Commun, 2006, 7(9):647-650. doi: 10.1016/j.catcom.2005.11.009 [5] 刘文丽. HZSM-5催化剂酸性调变对甲醇制烯烃反应性能的影响研究[D].银川: 宁夏大学, 2017.LIU Wen-li. Study on the acidity adjustment of HZSM-5[D]. Yinchuan: Ningxia University, 2017. [6] PARK S, BILIGETU T, WANG Y, NISHITOBA T, KONDO J N, YOKOI T. Acidic and catalytic properties of ZSM-5 zeolites with different Al distributions[J]. Catal Today, 2018, 303:64-70. doi: 10.1016/j.cattod.2017.07.022 [7] BILIGETU T, WANG Y, NISHITOBA T, OTOMO R, PARK S, MOCHIZUKI H, KONDO J N, TATSUMI T, YOKOI T. Al distribution and catalytic performance of ZSM-5 zeolites synthesized with various alcohols[J]. J Catal, 2017, 353:1-10. doi: 10.1016/j.jcat.2017.06.026 [8] HOLZINGER J, BEATO P, LUNDEGAARD L F, SKIBSTED J. Distribution of aluminum over the tetrahedral sites in ZSM-5 zeolites and their evolution after steam treatment[J]. J Phys Chem C, 2018, 122(27):15595-15613. doi: 10.1021/acs.jpcc.8b05277 [9] SANHOOB M A, MURAZA S, SHAFEI E N, YOKOI T, CHOI K H. The steam catalytic cracking of heavy naphtha (C12) to high octane naphtha over B-MFI zeolite[J]. Appl Catal B. Environ, 2017, 210:432-443. doi: 10.1016/j.apcatb.2017.04.001 [10] YANG Y S, SUN C, DU J M, YUE Y H, HUA W M, ZHANG C L, SHEN W, XU H L. The synthesis of endurable B-Al-ZSM-5 catalyst with tunable acidity for methanol to propylene reaction[J]. Catal Commun, 2012, 24:44-47. doi: 10.1016/j.catcom.2012.03.013 [11] LI C G, VIDAL-MOYA A, MIGUEL P J, DEDECEK J, BORONAT M, CORMA A. Selectively introducing acid sites in different confined positions in ZSM-5 and its catalytic implications[J]. ACS Catal, 2018, 8(8):7688-7697. doi: 10.1021/acscatal.8b02112 [12] EMEIS C A. Determination of integrated molar extinction coefficients for infrared absorption bands of pyridine adsorbed on solid acid catalysts[J]. J Catal, 1993, 141:347-354. doi: 10.1006/jcat.1993.1145 [13] HU Z J, ZHANG H B, WANG L, ZHANG H X, ZHANG Y H, XU H L, SHEN W, TANG Y. Highly stable boron-modified hierarchical nanocrystalline ZSM-5 zeolite for the methanol to propylene reaction[J]. Catal Sci Technol, 2014, 4:2981-2985. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=254505ade0d94a3000dfc23bc1551933 [14] DING C H, WANG X S, GUO X W, ZHANG S G. Characterization and catalytic alkylation of hydrothermally dealuminated nanoscale ZSM-5 zeolite catalyst[J]. Catal Commun, 2007, 9:487-493. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=c1924aee810170c226b98290aa322b16 [15] 周振垒, 李啄, 王博, 彭伟才, 李建青, 吴晋沪. ZSM-5的水热改性及其在合成气经二甲醚制汽油中的应用[J].燃料化学学报, 2013, 41(11):1349-1355. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rlhxxb201311011ZHOU Zhen-lei, LI Zhou, WANG Bo, PENG Wei-cai, LI Jian-qing, WU Jin-hu. Hydrothermal treatment of ZSM-5 and its application in syngas via DME[J]. J Fuel Chem Technol, 2013, 41(11):1349-1355. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rlhxxb201311011 [16] KANELLOPOULOS J, YNGER A, SCHWIEGER W, FREUDE D. Catalytic and multinuclear MAS NMR studies of a thermally treated zeolite ZSM-5[J]. J Catal, 2006, 237:416-425. doi: 10.1016/j.jcat.2005.11.030 [17] CHEN T H, WOUTERS B H, GROBET P J. Aluminium coordination in zeolite mordenite by27Al multiple quantum MAS NMR spectroscopy[J]. Eur J Inorg Chem, 2000, 2:281-285. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=3ff9d8f3ee73e3d0b250f1b5074d796f [18] YOKOI T, MOCHIZUKI H, NAMBA S, KONDO J N, TATSUMI T. Control of the Al distribution in the framework of ZSM-5 zeolite and its evaluation by solid-state NMR technique and catalytic properties[J]. J Phys Chem C, 2015, 119:15303-15315. doi: 10.1021/acs.jpcc.5b03289 [19] FU T J, MA Z, WANG Y J, SHAO J, MA Q, ZHANG C M, CUI L P, LI Z. Si/Al ratio induced structure evolution during desilication-recrystallization of silicalite-1 to synthesis nano-ZSM-5 catalyst for MTH reaction[J]. Fuel Process Technol, 2019, 194:106122. doi: 10.1016/j.fuproc.2019.106122 [20] LIU H, WANG H, XING A H, CHENG J H. Effect of Al distribution in MFI framework channels on the catalytic performance of ethane and ethylene aromatization[J]. J Phys Chem C, 2015, 119(27):15303-15315. doi: 10.1021/acs.jpcc.5b03289 [21] ERICHSEN M W, SVELLE S, OLSBYE U. The influence of catalyst acid strength on the methanol to hydrocarbons (MTH) reaction[J]. Catal Today, 2013, 215:216-223. doi: 10.1016/j.cattod.2013.03.017 [22] LIANG T Y, CHEN J L, QIN Z F, LI J F, WANG P F, WANG S, WANG G F, DONG M, FAN W B, WANG J G. Conversion of methanol to olefins over H-ZSM-5 zeolite:Reaction pathway is related to the framework aluminum siting[J]. ACS Catal, 2016, 6:7311-7325. doi: 10.1021/acscatal.6b01771 -





 下载:
下载: