Synthesis of hierarchical porous carbon loaded with chlorine and its mercury removal performance
-
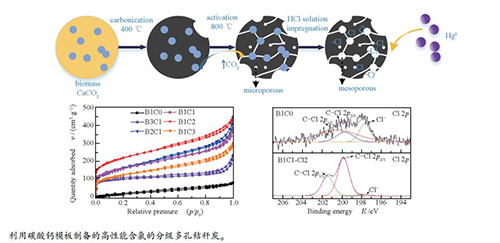
摘要: 以纳米碳酸钙为模板,水稻秸秆为碳前驱体,采用共热解法制备了负载氯的分级多孔生物质炭。在模拟烟气条件下,利用固定床实验台架研究了生物质碳材料对烟气中的单质汞(Hg0)的脱除性能。采用扫描电镜(SEM)、透射电镜(TEM)、N2吸附-脱附(BET)、程序升温脱附(Hg-TPD)以及X射线光电子能谱(XPS)等方法对材料进行表征。结果表明,盐酸浸渍不仅可去除模板产物生成多孔结构,并且有效地将氯负载到材料表面。负载氯的分级多孔炭B1C1-Cl2的比表面积和总孔容分别达到398.1 m2/g和0.4923 cm3/g。在120℃,空速(GHSV)为225000 h-1时,脱汞效率可达95%。多孔结构有利于气体扩散,高比表面积为材料提供了更多的反应位点,微孔-介孔内表面上的C-Cl共价键为脱汞的主要化学吸附活性位点。Abstract: Chlorine-loaded hierarchical porous bio-char was prepared by co-pyrolysis using nano-CaCO3 as template and rice straw as carbon precursor. The removal of mercury (Hg0) from flue gas by porous materials was studied on a fixed bed test bench with simulated flue gas. The materials were characterized by scanning electron microscopy (SEM), transmission electron microscopy (TEM), N2 adsorption-desorption (BET), temperature programmed desorption (Hg-TPD) and X-ray photoelectron spectroscopy (XPS). The results show that HCl impregnation not only removes the products on the template to form porous structures but also effectively loads chlorine onto the surface of the material. The specific surface area and total pore volume of B1C1-Cl2 are 398.1 m2/g and 0.4923 cm3/g, respectively. When the GHSV is 225000 h-1 at 120 ℃, the removal efficiency of Hg0 by chemical adsorption is up to 95%. The porous structure is beneficial to gas diffusion and the high specific surface area can provide more active sites. The covalent groups (C-Cl) participating in the Hg0 removal process are the dominant chemical adsorption sites on the inner micro-mesopore surface.
-
Key words:
- elemental mercury /
- porous bio-chars /
- chlorine /
- adsorption /
- flue gas
-
表 1 吸附剂的孔结构参数
Table 1 Pore structural parameters of sorbents
Sorbent ABET/(m2·g-1) Pore volume v/(cm3·g-1) R/nm vT vmic vmes B1C0 88.29 0.1285 0.0000 0.0954 5.82 B3C1 329.5 0.3613 0.1003 0.1517 4.38 B2C1 560.2 0.6859 0.0315 0.4348 4.89 B1C1 591.3 0.5952 0.0683 0.4548 4.31 B1C2 742.5 0.6972 0.1020 0.4768 3.75 B1C3 428.7 0.4655 0.0387 0.3368 4.34 B1C1-Cl0.5 447.1 0.5107 0.0539 0.3598 4.32 B1C1-Cl2 398.1 0.4923 0.0548 0.3011 4.94 B1C1-Cl5 364.5 0.4188 0.0726 0.2175 4.59 表 2 XPS谱图中相关官能团含量
Table 2 Related functional groups obtained from XPS spectra
Sample Atom content/% >Relative intensity/% C Cl Ca Cl- C-Cl 2p3/2 C-Cl 2p1/2 B1C0 90.5 0.40 0.07 23.08 19.20 57.71 B1C1-Cl2 88.5 1.50 0.10 2.36 63.68 33.95 B1C2-Cl2 68.93 1.25 0.17 6.13 59.68 34.18 -
[1] 高兰君, 王夫美, 吴撼明, 潘奕君, 沈伯雄. Ce-Co/KIT-6介孔材料的制备及其脱汞性能的研究[J].燃料化学学报, 2017, 45(8):1017-1024. doi: 10.3969/j.issn.0253-2409.2017.08.016GAO Lan-jun, WANG Fu-mei, WU Han-ming, PAN Yi-jun, SHEN Bo-xiong. Synthesis of mesoprous materials with Ce-Co/KIT-6 and its mercury removal performance[J]. J Fuel Chem Technol, 2017, 45(8):1017-1024. doi: 10.3969/j.issn.0253-2409.2017.08.016 [2] ZHOU Q, DUAN Y F, CHEN M M, LIU M, LU P. Studies on mercury adsorption species and equilibrium on activated carbon surface[J]. Energy Fuels, 2017, 31(12):14211-14218. doi: 10.1021/acs.energyfuels.7b02699 [3] LI H L, WU C Y, LI Y, ZHANG J Y. Superior activity of MnOx-CeO2/TiO2 catalyst for catalytic oxidation of elemental mercury at low flue gas temperatures[J]. Appl Catal B:Environ, 2012, 111/112:381-388. doi: 10.1016/j.apcatb.2011.10.021 [4] XU Y, ZENG X B, LUO G Q, ZHANG B, XU P, XU M H, YAO H. Chlorine-char composite synthesized by co-pyrolysis of biomass wastes and polyvinyl chloride for elemental mercury removal[J]. Fuel, 2016, 183:73-79. doi: 10.1016/j.fuel.2016.06.024 [5] LI G L, WANG S X, WANG F M, WU Q R, TANG Y, SHEN B X. Role of inherent active constituents on mercury adsorption capacity of chars from four solid wastes[J]. Chem Eng J, 2017, 307:544-552. doi: 10.1016/j.cej.2016.08.106 [6] WU J, LI Z, SONG Y. Preparation of biomass-derived hierarchically porous carbon/Co3O4 nanocomposites as anode materials for lithium-ion batteries[J]. J Alloy Compd, 2016, 656:745-752. doi: 10.1016/j.jallcom.2015.10.063 [7] XU B, HOU S S, ZHANG F, ZHANG F L, CAO G P, CHU M, YANG Y S. Nitrogen-doped mesoporous carbon derived from biopolymer as electrode material for supercapacitors[J]. J Electroanal Chem, 2014, 712:146-150. doi: 10.1016/j.jelechem.2013.11.020 [8] ISLAM M A, TAN I A W, BENHOURIA A, BEN A, ASIF M, HAMEED B H. Mesoporous and adsorptive properties of palm date seed activated carbon prepared via sequential hydrothermal carbonization and sodium hydroxide activation[J]. Chem Eng J, 2015, 270:187-195. doi: 10.1016/j.cej.2015.01.058 [9] ZHAO P F, GUO X, ZHENG C G. Removal of elemental mercury by iodine-modified rice husk ash sorbents[J]. J Environ Sci-China, 2010, 22(10):1629-1636. doi: 10.1016/S1001-0742(09)60299-0 [10] XU B, PENG L, WANG G, CAO G P, WU F. Easy synthesis of mesoporous carbon using nano-CaCO3 as template[J]. Carbon, 2010, 48(8):2377-2380. doi: 10.1016/j.carbon.2010.03.003 [11] CAO B, LIU H, XU B, LEI Y F, CHEN X H, SONG H H. Mesoporous soft carbon as an anode material for sodium ion batteries with superior rate and cycling performance[J]. J Mater Chem A, 2016, 4(17):6472-6478. doi: 10.1039/C6TA00950F [12] LI G L, SHEN B X, LI Y W, ZHAO B, WANG F M, HE C, WANG Y Y, ZHANG M. Removal of element mercury by medicine residue derived biochars in presence of various gas compositions[J]. J Hazard Mater, 2015, 298:162-169. doi: 10.1016/j.jhazmat.2015.05.031 [13] ZU G Q, SHEN J, ZOU L O, WANG F, WANG X D, ZHANG Y W, YAO X D. Nanocellulose-derived highly porous carbon aerogels for supercapacitors[J]. Carbon, 2015, 99:203-211. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=0c4fb30ff6283ac39dadd8c14ac600bf [14] KONG L J, LIU M X, DIAO Z H, CHEN D Y, CHANG X Y, XIONG Y. Coupling template nanocasting and self-activation for fabrication of nanoporous carbon[J]. Sci Rep, 2016, 6:38176. doi: 10.1038/srep38176 [15] WANG T, WU J W, ZHANG Y S, LIU J, SUI Z F, ZHANG H C, CHEN W Y, NORRIS P, PAN W P. Increasing the chlorine active sites in the micropores of biochar for improved mercury adsorption[J]. Fuel, 2018, 229:60-67. doi: 10.1016/j.fuel.2018.05.028 [16] GHORISHI S B, KEENEY R M, SERRE S D. Development of a Cl-impregnated activated carbon for entrained-flow capture of elemental mercury[J]. Environ Sci Technol, 2002, 36(20):4454-4459. doi: 10.1021/es0255608 [17] LI G L, WANG S X, WU Q R, WANG F Y, SHEN B X. Mercury sorption study of halides modified bio-chars derived from cotton straw[J]. Chem Eng J, 2016, 302:305-313. doi: 10.1016/j.cej.2016.05.045 [18] IRIARTE-VELASCO U, SIERRA I, ZUDAIRE L, AYASTUY J L. Preparation of a porous biochar from the acid activation of pork bones[J]. Food Bioprod Process, 2016, 98:341-353. doi: 10.1016/j.fbp.2016.03.003 [19] LI G L, WANG S X, WU Q G, WANG, F Y, DING D, SHEN B X. Mechanism identification of temperature influence on mercury adsorption capacity of different halides modified bio-chars[J]. Chem Eng J, 2017, 315:251-261. doi: 10.1016/j.cej.2017.01.030 [20] LI G L, SHEN B X, WANG Y, YUE S J, XI Y Q, AN M D, REN K K. Comparative study of element mercury removal by three bio-chars from various solid wastes[J]. Fuel, 2015, 145:189-195. doi: 10.1016/j.fuel.2014.12.083 [21] SANO A, TAKAOKA M, SHIOTA K. Vapor-phase elemental mercury adsorption by activated carbon co-impregnated with sulfur and chlorine[J]. Chem Eng J, 2017, 315:598-607. doi: 10.1016/j.cej.2017.01.035 [22] LEE S F, SEO Y C, JURNG J, LEE T G. Removal of gas-phase elemental mercury by iodine- and chlorine-impregnated activated carbons[J]. Atmos Environ, 2004, 38(29):4887-4893. doi: 10.1016/j.atmosenv.2004.05.043 [23] XU Y, ZENG X B, ZHANG B, ZHU X Q, ZHOU M L, ZOU R J, SUN P, LUO G Q, YAO H. Experiment and kinetic study of elemental mercury adsorption over a novel chlorinated sorbent derived from coal and waste polyvinyl chloride[J]. Energy Fuels, 2016, 30(12):10635-10642. doi: 10.1021/acs.energyfuels.6b01372 [24] XU Y, LUO G Q, HE S W, DENG F F, PANG Q, XU Y Q, YAO H. Efficient removal of elemental mercury by magnetic chlorinated biochars derived from co-pyrolysis of Fe(NO3)3-laden wood and polyvinyl chloride waste[J]. Fuel, 2019, 239:982-990. doi: 10.1016/j.fuel.2018.11.102 [25] 赵鹏飞, 郭欣, 郑楚光.活性炭及氯改性活性炭吸附单质汞的机制研究[J].中国电机工程学报, 2010, 30(23):40-44. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgdjgcxb201023007ZHAO Peng-fei, GUO Xin, ZHENG Chu-guang. Investigating the mechanism of elemental mercury binding on activated carbon and chlorine-embedded activated carbon[J]. Proc CSEE, 2010, 30(23):40-44. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgdjgcxb201023007 -





 下载:
下载: