Influence of cerium sources on CuO/CeO2 catalysts for hydrogen production from steam reforming of methanol
-
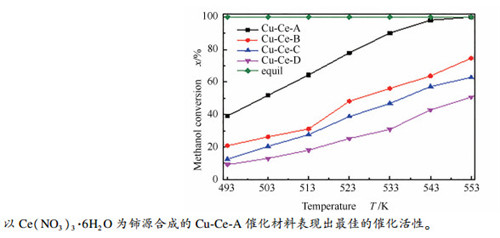
摘要: 采用沉淀法合成了CeO2载体,再经浸渍法负载活性组分得到CuO/CeO2催化材料,探究了铈源(Ce(NO3)3·6H2O、CeCl3·6H2O、Ce(NH4)2(NO3)6、Ce(SO4)2·4H2O)对CuO/CeO2催化性能的影响。通过采用XRD、SEM、N2O滴定、BET和H2-TPR等表征手段对催化材料的结构和性质研究发现,四种铈源合成的CuO/CeO2催化材料在Cu比表面积、还原性能以及活性组分和载体间的相互作用方面存在着明显差别。其中,由Ce(NO3)3·6H2O合成的CuO/CeO2催化材料的Cu比表面积较大,CuO还原温度较低,CeO2载体与CuO之间相互作用较强,在甲醇水蒸气重整反应过程中,表现出较佳的催化活性,在反应温度为553 K,水醇比n(H2O)/n(MeOH)为1.2,甲醇水蒸气气体空速(GHSV)为1760 h-1时,甲醇的转化率为100%,重整气中CO摩尔含量为0.84%。Abstract: CeO2 support was synthesized by precipitation method and used to prepare CuO/CeO2 catalyst using impregnation method. The effects of cerium sources (Ce(NO3)3·6H2O, CeCl3·6H2O, Ce(NH4)2(NO3)6 and Ce(SO4)2·4H2O) on the catalytic performance of CuO/CeO2 catalyst were investigated. XRD, SEM, N2O titration, BET and H2-TPR were used to study the structure and properties of the catalysts. The CuO/CeO2 catalysts with different cerium sources have obvious differences in Cu specific surface area, reduction performance and interaction between active component and support. Moreover, CuO/CeO2 catalyst prepared with Ce(NO3)3·6H2O has large Cu specific surface area, low reduction temperature and strong interaction between CeO2 support and CuO and shows better catalytic activity in methanol steam reforming. The methanol conversion is 100% at reaction temperature of 553 K, water/methanol ratio in feed of 1.2 and methanol water gas hourly space velocity of 1760 h-1. Besides, the CO molar content in reformed gas is 0.84%.
-
Key words:
- method of precipitation /
- cerium source /
- methanol steam reforming /
- hydrogen
-
表 1 催化剂表面的物化性质和产氢速率
Table 1 Physical properties of the synthesized catalysts and hydrogen production rate in methanol steam reforming
Catalyst ABET
/(m2·g-1)Pore volume
v/(cm3·g-1)Cu dispersion /% Cu surface area
A /(m2·g-1)H2 production ratea
/(μmol·kg-1·s-1)CeO2-A 37.4 0.10 - - - CeO2-B 59.1 0.15 - - - CeO2-C 96.9 0.04 - - - CeO2-D 110.3 0.05 - - - Cu-Ce-A 21.9 0.09 15 8.8 16941 Cu-Ce-B 54.6 0.14 13 7.2 14753 Cu-Ce-C 81.6 0.03 8 4.4 9329 Cu-Ce-D 83.1 0.04 2 1.2 7874 aH2 production rate was calculated when temperature is 553K, n(H2O)/n(MeOH)=1.2:1, GHSV=1760h-1 表 2 催化剂还原峰位置
Table 2 Reduction peak position of the catalysts
Catalyst Peak position t/℃ peak 1 peak 2 Cu-Ce-A 178 222 Cu-Ce-B 182 253 Cu-Ce-C 195 227 Cu-Ce-D 200 223 表 3 催化材料的甲醇转化率和CO摩尔含量对比
Table 3 Comparison of methanol conversion and CO molar content
-
[1] 苏石龙, 张磊, 张艳, 雷俊腾, 桂建舟, 刘丹, 刘道胜, 潘立卫.千瓦级PEMFC甲醇水蒸气重整制氢过程热力学模拟[J].石油化工高等学校学报, 2015, 28(2):19-25. doi: 10.3969/j.issn.1006-396X.2015.02.004SU Shi-long, ZHANG Lei, ZHANG Yan, LEI Jun-teng, GUI Jian-zhou, LIU Dan, LIU Dao-sheng, PAN Li-wei. Thermodynamic simulation for hydrogen production in the methanol steam reforming system of kilowatt PEMFC[J]. J Petrochem Univ, 2015, 28(2):19-25. doi: 10.3969/j.issn.1006-396X.2015.02.004 [2] YANG S C, SU W N, LIN S D, RICK J, HWANG B J. Preparation of highly dispersed catalytic Cu from rod-like CuO-CeO2 mixed metal oxides:Suitable for applications in high performance methanol steam reforming[J]. Catal Sci Technol, 2012, 2(4):807-812. doi: 10.1039/c2cy00330a [3] HE J P, YANG Z X, ZHANG L, LI Y, PAN L W. Cu supported on ZnAl-LDHs precursor prepared by in-situ synthesis method onγ -Al2O3 as catalytic material with high catalytic activity for methanol steam reforming[J]. Int J Hydrogen Energy, 2017, 42(15):9930-9937. doi: 10.1016/j.ijhydene.2017.01.229 [4] ZHANG L, PAN L W, NI C J, SUN T J, ZHAO S S, WANG S D, WANG A J, HU Y K. CeO2-ZrO2-promoted CuO/ZnO catalyst for methanol steam reforming[J]. Int J Hydrogen Energy, 2013, 38(11):4397-4406. doi: 10.1016/j.ijhydene.2013.01.053 [5] LIU N, YUAN Z S, WANG C W, WANG S D, ZHANG C X, WANG S J. The role of CeO2-ZrO2 as support in the ZnO-ZnCr2O4 catalysts for autothermal reforming of methanol[J]. Fuel Process Technol, 2008, 89(6):574-581. doi: 10.1016/j.fuproc.2007.11.029 [6] PATEL S, PANT K K. Selective production of hydrogen via oxidative steam reforming of methanol using Cu-Zn-Ce-Al oxide catalysts[J]. Chem Eng Sci, 2007, 62(18/20):5436-5443. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=c3881db91f418532b85f2cd5f55273fb [7] HAO C, ANDOLINA C M, LI J, CURNAN M T, SAIDI W A, ZHOU G W, YANG J C, VESER G. Dependence of H2 and CO2 selectivity on Cu oxidation state during partial oxidation of methanol on Cu/ZnO[J]. Appl Catal A:Gen, 2018, 556:64-72. doi: 10.1016/j.apcata.2018.02.028 [8] MA Y F, GUAN G Q, PHANTHONG P, LI X M, CAO J, HAO X G, WANG Z D, ABUDULA A. Steam reforming of methanol for hydrogen production over nanostructured wire-like molybdenum carbide catalyst[J]. Int J Hydrogen Energy, 2014, 39(33):18803-18811. doi: 10.1016/j.ijhydene.2014.09.062 [9] 李永峰, 林维明, 余林.两种甲醇水蒸气重整制氢催化剂的研究[J].燃料化学学报, 2004, 32(5):106-110. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract16649.shtmlLI Yong-feng, LIN Wei-ming, YU Lin. Study on two hydrogen production catalysts for methanol steam reforming[J]. J Fuel Chem Technol, 2004, 32(5):106-110. http://manu60.magtech.com.cn/rlhxxb/CN/abstract/abstract16649.shtml [10] 刘玉娟, 许骥, 佟宇飞, 张娜, 张磊, 刘道胜, 韩蛟, 张财顺.氧化铈纳米材料合成方法的研究进展[J].辽宁石油化工大学学报, 2017, 37(5):8-12. doi: 10.3969/j.issn.1672-6952.2017.05.002LIU Yu-juan, XU Ji, TONG Yu-fei, ZHANG Na, ZHANG Lei, LIU Dao-sheng, HAN Jiao, ZHANG Cai-shun. Progress in research of the synthesis methods of nanometer ceria[J]. J Liaoning Shihua Univ, 2017, 37(5):8-12. doi: 10.3969/j.issn.1672-6952.2017.05.002 [11] LIU Y, HAYAKAWA T, TSUNODA T, SUZUKI K, HAMAKAWA S, MURATA K, NSHIOZAKI R, ISHII T, KUMAGAI M. Steam reforming of methanol over Cu/CeO2 catalysts studied in comparison with Cu/ZnO and Cu/Zn(Al)O catalysts[J]. Top Catal, 2003, 22(3/4):205-213. doi: 10.1023/A:1023519802373 [12] SUN C W, LI H, ZHANG H R, WANG Z X, CHEN L Q. Controlled synthesis of CeO2 nanorods by a solvothermal method[J]. Nanotechnol, 2005, 16(9):1454-1463. doi: 10.1088/0957-4484/16/9/006 [13] ZHANG C Y, CHU W, CHEN F, LI L, JIANG R Y, YAN J L. Effects of cerium precursors on surface properties of mesoporous CeMnOx catalysts for toluene combustion[J]. J Rare Earths, 2019, 38(1):70-75. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=zgxtxb-e202001010 [14] 刘玉娟, 王东哲, 张磊, 王宏浩, 陈琳, 刘道胜, 韩蛟, 张财顺.载体焙烧气氛对甲醇水蒸气重整制氢CuO/CeO2催化剂的影响[J].燃料化学学报, 2018, 46(8):992-999. doi: 10.3969/j.issn.0253-2409.2018.08.011LIU Yu-juan, WANG Dong-zhe, ZHANG Lei, WANG Hong-hao, CHEN Lin, LIU Dao-sheng, HAN Jiao, ZHANG Cai-shun. Effect of support calcination atmospheres on the activity of CuO/CeO2 catalysts for methanol steam reforming[J]. J Fuel Chem Technol, 2018, 46(8):992-999. doi: 10.3969/j.issn.0253-2409.2018.08.011 [15] 杨淑倩, 贺建平, 张娜, 隋晓伟, 张磊, 杨占旭.稀土掺杂改性Cu/Zn-Al水滑石衍生催化剂对甲醇水蒸气重整制氢性能的影响[J].燃料化学学报, 2018, 46(2):179-188. doi: 10.3969/j.issn.0253-2409.2018.02.007YANG Shu-qian, HE Jian-ping, ZHANG Na, SUI Xiao-wei, ZHANG Lei, YANG Zhan-xu. Effect of rare-earth element modification on the performance of Cu/ZnAl catalysts derived from hydrotalcite precursor in methanol steam reforming[J]. J Fuel Chem Technol, 2018, 46(2):179-188. doi: 10.3969/j.issn.0253-2409.2018.02.007 [16] YANG S Q, ZHOU F, LIU Y J, ZHANG L, CHEN Y, WANG H H, TIAN Y, ZHANG C S, LIU D S. Morphology effect of ceria on the performance of CuO/CeO2 catalysts for hydrogen production by methanol steam reforming[J]. Int J Hydrogen Energy, 2019, 44(14):7252-7261. doi: 10.1016/j.ijhydene.2019.01.254 [17] ZHANG Y J, CHEN C Q, ZHANG Y Y, LIN Q, LOU B Y, ZHENG G C, ZHENG Q. Highly active Y-promoted CuO/ZrO2 catalysts for the production of hydrogen through water-gas shift reaction[J]. J Fuel Chem Technol, 2017, 45(9):1137-1149. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=rlhxxb201709015 [18] ZHANG Y J, CHEN C Q, LIN X Y, LI D L, CHEN X H, ZHAN Y Y, ZHENG Q. CuO/ZrO2 catalysts for water-gas shift reaction:Nature of catalytically active copper species[J]. Int J Hydrogen Energy, 2014, 39(8):3746-3754. doi: 10.1016/j.ijhydene.2013.12.161 [19] XIE H M, DU Q X, LI H, ZHOU G L, CHEN S M, JIAO Z J, REN J M. Catalytic combustion of volatile aromatic compounds over CuO-CeO2 catalyst[J]. Korean J Chem Eng, 2017, 34(7):1944-1951. doi: 10.1007/s11814-017-0111-4 [20] BERA P, PRIOLKAR K R, SARODE P R, HEGDE M S, EMURA S, KUMASHIRO R, LALLA N P. Structural investigation of combustion synthesized Cu/CeO2 catalysts by EXAFS and other physical techniques:Formation of a Ce1-x CuxO2-δ solid solution[J]. Chem Mater, 2002, 14(8):3591-3601. doi: 10.1021/cm0201706 [21] AVGOUROPOULOS G, IOANNIDES T, MATRALIS H. Influence of the preparation method on the performance of CuO-CeO2 catalysts for the selective oxidation of CO[J]. Appl Catal B:Environ, 2005, 56(1/2):87-93. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=3887d474fb479182a25d534d11f5c3ba [22] LUO M F, ZHONG Y J, YUAN X X, ZHENG X M. TPR and TPD studies of CuO/CeO2 catalysts for low temperature CO oxidation[J]. Appl Catal A:Gen, 1997, 162(1):121-131. http://www.ingentaconnect.com/content/els/0926860x/1997/00000162/00000001/art00089 [23] 张磊, 潘立卫, 倪长军, 孙天军, 赵生生, 王树东, 胡永康, 王安杰.沉淀温度对CuO/ZnO/CeO2/ZrO2甲醇水蒸气重整制氢催化剂性能的影响[J].催化学报, 2012, 33(12):1958-1964. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=cuihuaxb201212012ZHANG Lei, PAN Li-wei, NI Chang-jun, SUN Tian-jun, ZHAO Sheng-sheng, WANG Shu-dong, HU Yong-kang, WANG An-jie. Effect of precipitation temperature on the performance of CuO/ZnO/CeO2/ZrO2 catalyst for methanol steam reforming[J].Chin J Catal, 2012, 33(12):1958-1964. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=cuihuaxb201212012 [24] 刘玉娟, 王东哲, 张磊, 白金, 陈琳, 刘道胜. CeO2形貌对甲醇水蒸汽重整CuO/CeO2催化剂的影响[J].精细化工, 2018, 35(12):71-77+112. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=jxhg201812011LIU Yu-juan, WANG Dong-zhe, ZHANG Lei, BAI Jin, CHEN Lin, LIU Dao-sheng. Effect of CeO2 morphology on CuO/CeO2 catalyst for methanol steam reforming[J]. Fine Chem, 2018, 35(12):71-77+112. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=jxhg201812011 [25] 赵红霞, 李永红, 杨成, 王秀芝, 任杰, 孙予罕. Fe改性工业Cu/Zn/Al催化剂上甲醇水蒸气重整反应制氢的研究[J].燃料化学学报, 2001, 29:137-139. doi: 10.3969/j.issn.0253-2409.2001.z1.043ZHAO Hong-xia, LI Yong-hong, YANG Cheng, WANG Xiu-zhi, REN Jie, SUN Yu-han. Hydrogen production by steam reforming of methanol on Fe modified Cu/Zn/Al catalysts[J]. J Fuel Chem Technol, 2001, 29:137-139. doi: 10.3969/j.issn.0253-2409.2001.z1.043 [26] 王路存, 刘永梅, 曹勇, 吴贵升, 姚成漳, 戴维林, 贺鹤勇, 范康年.草酸盐固相化学法制备高性能Cu/ZnO甲醇水蒸气重整催化剂[J].化学学报, 2007, 65(2):173-176. doi: 10.3321/j.issn:0567-7351.2007.02.016WANG Lu-cun, LIU Yong-mei, CAO Yong, WU Gui-sheng, YAO Cheng-zhang, DAI Wei-lin, HE He-yong, FAN Kang-nian. Preparation of high performance by oxalate solid phase chemical Cu/ZnO methanol steam reforming catalyst[J]. Acta Chim Sin, 2007, 65(2):173-176. doi: 10.3321/j.issn:0567-7351.2007.02.016 -





 下载:
下载: