Fe-PILC for selective catalytic reduction of NO by propene under lean-burn conditions
-
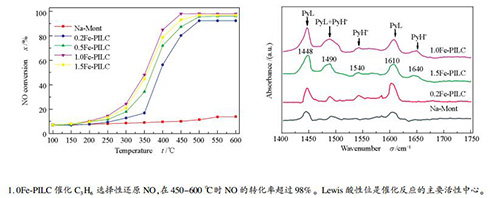
摘要: 采用羟基铁离子柱撑Na-Mont制备了铁柱撑黏土催化剂(Fe-PILC),铁物种作为柱撑成分,同时充当活性组分,研究其在贫燃条件下催化丙烯选择性还原NO的特性。通过XRD、N2吸附-脱附、H2-TPR、UV-vis、Py-FTIR等方法对催化剂进行物理化学性质表征,进一步研究其反应机理。研究表明,1.0Fe-PILC在450-600 ℃时NO的转化率超过98%,N2的选择性可达97%以上,且抗水蒸气和SO2的能力较强。XRD和N2吸附-脱附研究表明,Fe-PILC中铁物种柱撑进入Na-Mont层间,使催化剂的比表面积和孔容增大。H2-TPR研究表明,Fe-PILC在400 ℃左右还原能力较强,主要体现为Fe3+→Fe2+的还原。UV-vis研究表明,Fe-PILC的脱硝活性与铁氧低聚物种FexOy呈正相关。Py-FTIR研究表明,Fe-PILC表面同时含有Lewis酸和Brønsted酸性位,Lewis酸性位是C3H6与NO进行催化反应的主要活性中心。Abstract: Iron-pillared clays (Fe-PILC) were prepared by ion-exchange method and the iron species act as the pillaring components and active components and their performances for selective catalytic reduction of NO by propene were investigated under lean-burn conditions. XRD, N2 adsorption/desorption, H2-TPR, UV-vis, Py-FTIR, etc were used to characterize the catalysts. The results showed that 1.0Fe-PILC reduced more than 98% of NO at 450-600℃ and the selectivity for N2 reached 97%. The catalytic activity of the 1.0Fe-PILC was slightly influenced by water vapor and SO2. XRD and N2 adsorption/desorption characterization results showed that the iron species entered the Na-Mont interlayer and formed much larger specific surface area and pore volume. H2-TPR results indicated that Fe-PILC had a strong reduction ability at about 400 ℃, which represented the reduction of Fe3+→Fe2+. UV-vis results showed that the denitrification activity of Fe-PILC was positively correlated with the iron oxide oligomer FexOy. Py-FTIR results revealed that Lewis acid and Brønsted acid sites formed on the Fe-PILC surface. The main catalytic activity center of C3H6 and NO reaction was the Lewis acid site.
-
Key words:
- lean-burn conditions /
- Fe-PILC /
- C3H6 /
- selective catalytic reduction /
- NO
-
表 1 不同催化剂的物理特性
Table 1 Physical properties of different catalysts
Catalyst Fe w/(mg·g-1)a ABET/(m2·g-1) Pore volume v/(cm3·g-1) Pore diameter d/nm Na-Mont - 48.6 0.116 9.58 0.2Fe-PILC 142.91 92.0 0.233 8.11 1.0Fe-PILC 180.25 194.7 0.271 6.26 1.5Fe-PILC 190.49 181.2 0.228 5.56 a: ICP analysis results 表 2 不同催化剂的B酸和L酸含量
Table 2 Brønsted and Lewis acid content of different catalysts
Sample 150 ℃ desorption /(μmol·g-1) 300 ℃ desorption/(μmol·g-1) B L B L Na-Mont 0 45.27 0 23.19 0.2Fe-PILC 2.68 49.01 1.06 35.96 1.0Fe-PILC 4.60 108.87 1.25 58.59 1.5Fe-PILC 2.88 84.63 1.14 52.45 -
[1] KOTSIFA A, KONDARIDES D I, VERYKIOS X E. A comparative study of the selective catalytic reduction of NO by propylene over supported Pt and Rh catalysts[J]. Appl Catal B:Environ, 2008, 80(3):260-270. http://cn.bing.com/academic/profile?id=37ac523f2e17427bc7f17dac4a2c5a5c&encoded=0&v=paper_preview&mkt=zh-cn [2] 石新雨, 储伟.载体及银含量等参数对丙烯选择还原NO用银基催化剂性能的影响[J].天然气化工, 2009, 34(4):47-52. doi: 10.3969/j.issn.1001-9219.2009.04.012SHI Xin-yu, CHU Wei. Effect of support and Ag loading on the performance of silver-based catalysts for selective catalytic reduction of NO by C3H6[J]. J Nat Gas Chem, 2009, 34(4):47-52. doi: 10.3969/j.issn.1001-9219.2009.04.012 [3] XIE S, WANG J, HE H. Poisoning effect of sulphate on the selective catalytic reduction of NOx by C3H6 over Ag-Pd/Al2O3[J]. J Mol Catal A:Chem, 2007, 266(1/2):166-172. [4] ZHANG R, VILLANUEVA A, ALAMDARI H, ET AL. SCR of NO by propene over nanoscale LaMn1-xCuxO3perovskites[J]. Appl Catal A:Gen, 2006, 307(1):85-97. doi: 10.1016/j.apcata.2006.03.019 [5] LIU Z M, LI J H, HAO J M. Selective catalytic reduction of NOx with propene over SnO2/Al2O3 catalyst[J]. Chem Eng J, 2010, 165(2):420-425. doi: 10.1016/j.cej.2010.09.009 [6] IWAMOTO M, YAHIRO H, YU U Y. Selective reduction of NO by lower hydrocarbons in the presence of O2 and SO2 over copper ion-exchanged zeolites[J]. Catal, 1990, 32(6):p430-433. http://cn.bing.com/academic/profile?id=cf5d3c3705d15b224ce43f4bb7a701ab&encoded=0&v=paper_preview&mkt=zh-cn [7] LI L, GUAN N. HC-SCR reaction pathways on ion exchanged ZSM-5 catalysts[J]. Microporous Mesoporous Mater, 2009, 117(1/2):450-457. http://cn.bing.com/academic/profile?id=e18a524855d1e95c7c3fa283e97d7f68&encoded=0&v=paper_preview&mkt=zh-cn [8] KOMVOKIS V G, ILIOPOULOU E F, VASALOS I A, TRIANTAFYLLIDIS K S, MARSHALL C L. Development of optimized Cu-ZSM-5 deNOx catalytic materials both for HC-SCR applications and as FCC catalytic additives[J]. Appl Catal A:Gen, 2007, 325(2):345-352. doi: 10.1016/j.apcata.2007.02.035 [9] CHAE H J, NAM I-S, HAM S W, HONG S B. Physicochemical characteristics of pillared interlayered clays[J]. Catal Today, 2001, 68(1):31-40. http://cn.bing.com/academic/profile?id=72eeafc5862a70be1f5f451a34ec1a16&encoded=0&v=paper_preview&mkt=zh-cn [10] VALVERDE J L, LUCAS A D, SÁNCHEZ P, DORADO F, ROMERO A. Cation exchanged and impregnated Ti-pillared clays for selective catalytic reduction of NOx by propylene[J]. Appl Catal B:Environ, 2003, 43(1):43-56. doi: 10.1016/S0926-3373(02)00274-6 [11] DORADO F, LUCAS A D, GARCÍA P B, VALVERDE J L, ROMERO A. Preparation of Cu-ion-exchanged Fe-PILCs for the SCR of NO by propene[J]. Appl Catal B:Environ, 2006, 65(3/4):175-184. [12] BURCH R, MILLINGTON P J, WALKER A P. Mechanism of the selective reduction of nitrogen monoxide on platinum-based catalysts in the presence of excess oxygen[J]. Appl Catal:B Environ, 1994, 4(1):65-94. doi: 10.1016/0926-3373(94)00014-X [13] 钱文燕, 苏亚欣, 杨溪, 袁旻昊, 邓文义, 赵兵涛. Fe/Al-PILC催化C3H6选择性还原NO的实验研究[J].燃料化学学报, 2017, 45(12):1499-1507. doi: 10.3969/j.issn.0253-2409.2017.12.012QIAN Wen-yan, SU Ya-xin, YANG Xi, YUAN Min-hao, DENG Wen-yi, ZHAO Bing-tao. Experimental study on selective catalytic reduction of NO with propene over iron based catalysts supported on aluminum pillared clays[J]. J Fuel Chem Technol, 2017, 45(12):1499-1507. doi: 10.3969/j.issn.0253-2409.2017.12.012 [14] YANG T T, BI H T, CHENG X X. Effects of O2, CO2 and H2O on NOx adsorption and selective catalytic reduction over Fe/ZSM-5[J]. Appl Catal B:Environ, 2011, 102(1/2):163-171. https://www.researchgate.net/publication/257370588_An_investigation_of_the_surface_intermediates_of_H2-SCR_of_NOx_over_PtH-FER [15] LÓNYI F, SOLT H E, VALYON J, BOIX A, GUTIERREZ L B. The SCR of NO with methane over In, H-and Co, In, H-ZSM-5 catalysts:The promotional effect of cobalt[J]. Appl Catal B:Environ, 2012, s117-118(1):212-223. http://cn.bing.com/academic/profile?id=13bad5f8995c6fd856512ed0247ad793&encoded=0&v=paper_preview&mkt=zh-cn [16] CHEN S W, YAN X L, WANG Y, CHEN S W, YAN X L, WANG Y, CHEN J Q, PAN D H, MA J H, LI R F. Effect of SO2 on Co sites for NO-SCR by CH4 over Co-Beta[J]. Catal Today, 175(2011):12-17. doi: 10.1016/j.cattod.2011.05.024 [17] VALVERDE J L, DORADO F, SÁNCHEZ P, ISAAC ASENCIO A, ROMERO A. Synthesis and characterization of Cu-TiPILCs for selective catalytic reduction of NO by propylene in the presence of oxygen and H2O:Influence of the calcination temperature, the copper content, and the cation promoter (Ce/Ag)[J]. J Pharm Res Clin Pract, 2004, 42(17):3871-3880. http://cn.bing.com/academic/profile?id=72e7142534ae43ac925104e2af9224d9&encoded=0&v=paper_preview&mkt=zh-cn [18] ZHOU H, SU Y X, LIAO W Y, ZHONG F C. NO reduction by propane over monolithic cordierite-based Fe/Al2O3 catalyst:Reaction mechanism and effect of H2O/SO2[J]. Fuel, 2016, 182:352-360. doi: 10.1016/j.fuel.2016.05.116 [19] MURPHY P J, POSNER A M, QUIRK J P. Characterization of partially neutralized ferric chloride solutions[J]. J Colloid Interface Sci, 1976, 56(2):270-283. doi: 10.1016/0021-9797(76)90253-8 [20] BOTTERO J Y, MANCEAU A, VILLIERAS F, TCHOUBAR D. Structure and mechanisms of formation of iron oxide hydroxide (chloride) polymers[J]. Langmuir, 1994, 10(1):316-319. doi: 10.1021/la00013a046 [21] BRUNAUER S, DEMING L S, DEMING W E, TELLER E. On a theory of the van der waals adsorption of gases[J]. J Am Chem Soc, 1940, 62(7):1723-1732. doi: 10.1021/ja01864a025 [22] SING K S W. Reporting physisorption data for gas/solid systems-with special reference to the determination of surface area and porosity[J]. Pure Appl Chem, 1985, 57(4):603-619. doi: 10.1351/pac198557040603 [23] 沈伯雄, 马宏卿, 杨晓燕, 姚燕. Mn-CeOx/Ti-PILC的制备、表征及脱硝性能研究[J].燃料化学学报, 2012, 40(5):615-620. doi: 10.3969/j.issn.0253-2409.2012.05.017SHEN Bo-xiong, MA Hong-qing, YANG Xiao-yan, YAO Yan. Study on preparation, characterization and de-NO activity of Mn-CeOx/Ti-PILC[J]. J Fuel Chem Technol, 2012, 40(5):615-620. doi: 10.3969/j.issn.0253-2409.2012.05.017 [24] HATIPOǦLU M, HELVACI C, CHAMBERLAIN S C, BABALIK H. Mineralogical characteristics of unusual "Anatolian" diaspore (zultanite) crystals from the İlbirdaǧi diasporite deposit, Turkey[J]. J Afr Earth Sci, 2010, 57(5):525-541. doi: 10.1016/j.jafrearsci.2010.01.002 [25] EMBAID B P, BIOMORGI J G, GONZALEZ-JIMENEZ F, PÉREZ-ZURITA M J, SCOTT C E. USING Fe-PILC as catalyst[J]. Appl Catal A:Gen, 2011, 400(1):166-170. http://cn.bing.com/academic/profile?id=2e9074dc62e37c3eb2d415d56b4c9c68&encoded=0&v=paper_preview&mkt=zh-cn [26] CHEN H Y, SACHTLER W M H. Activity and durability of Fe/ZSM-5 catalysts for lean burn NOx, reduction in the presence of water vapor[J]. Catal Today, 1998, 42(1/2):73-83. [27] LONG R Q, YANG R T. Selective catalytic reduction of nitrogen oxides by ammonia over Fe3+-exchanged TiO2-pillared clay catalysts[J]. J Catal, 1999, 186(2):254-268. doi: 10.1006/jcat.1999.2558 [28] CHENG D G, CHEN F, ZHAN X. Characterization of iron-containing AlPO-5 as a stable catalyst for selective catalytic reduction of N2O with CH4 in the presence of steam[J]. Appl Catal A:Gen, 2012, 435/436(17):27-31. http://cn.bing.com/academic/profile?id=e9b99c996de57a8dd6e31124abf2af57&encoded=0&v=paper_preview&mkt=zh-cn [29] KUMAR M S, SCHWIDDER M, GRVNERT W, BRVCKNER A. On the nature of different iron sites and their catalytic role in Fe-ZSM-5 DeNOx catalysts:New insights by a combined EPR and UV/VIS spectroscopic approach[J]. J Catal, 2004, 227(2):384-397. doi: 10.1016/j.jcat.2004.08.003 [30] PÉREZ-RAMIÍREZ J, KUMAR M S, BRVCKNER A. Reduction of N2O with CO over FeMFI zeolites:Influence of the preparation method on the iron species and catalytic behavior[J]. J Catal, 2004, 223(1):13-27. doi: 10.1016/j.jcat.2004.01.007 [31] NEDYALKOVA R, SHWAN S, SKOGLUNDH M, OLSSON L. Improved low-temperature SCR activity for Fe-BEA catalysts by H2-pretreatment[J]. Appl Catal B:Environ, 2013, s138/139(21):373-380. [32] BARZETTI T, SELLI E, MOSCOTTI D, FORNI L. Pyridine and ammonia as probes for FT-IR analysis of solid acid catalysts[J]. J Chem Soc, Faraday Trans, 1996, 92(8):1401-1407. doi: 10.1039/ft9969201401 [33] DATKA J, TUREK A M, JEHNG J M, WACHS I E. Acidic properties of supported niobium oxide catalysts:An infrared spectroscopy investigation[J]. J Catal, 1992, 135(135):186-199. doi: 10.1016-0021-9517(92)90279-Q/ [34] LONG R Q, YANG R T. Selective catalytic reduction of nitrogen oxides by ammonia over Fe3+-exchanged TiO2-pillared clay catalysts[J]. J Catal, 1999, 186(2):254-268. doi: 10.1006/jcat.1999.2558 [35] HE M Y, LIU Z, MIN E. Acidic and hydrocarbon catalytic properties of pillared clay[J]. Catal Today, 1988, 2(2):321-338. doi: 10.1016-0920-5861(88)85013-2/ [36] SATSUMA A, SHIMIZU K I. In situ FT/IR study of selective catalytic reduction of NO over alumina-based catalysts[J]. Pro Energy Combust Sci, 2003, 29(1):71-84. doi: 10.1016/S0360-1285(02)00033-3 [37] LI J, ZHU Y, KE R, HAO J. Improvement of catalytic activity and sulfur-resistance of Ag/TiO2-Al2O3for NO reduction with propene under lean burn conditions[J]. Appl Catal B:Environ, 2008, 80(3):202-213. -





 下载:
下载: