Effect of synthesis solution pH of Co/γ-Al2O3 catalyst on its catalytic properties for methane conversion to syngas
-
Abstract: The cobalt nanoparticles over γ-Al2O3 support were prepared via chemical reduction of CoCl2·6H2O using NaBH4 with various values of pH in the range of 11.92-13.80. Synthesized catalysts were studied through X-ray diffraction (XRD), N2 adsorption/desorption (BET), H2-temperature programmed reduction (H2-TPR), H2-chemisorption, O2 pulse titration and temperature programmed oxidation (TPO) methods. Obtained results exhibited the synthesis solution pH showed a significant influence on the activity and selectivity in partial oxidation of methane reaction. The methane conversion, CO selectivity and H2 yield were enhanced by increasing of the synthesis solution pH. Compared to other catalysts, the catalyst that synthesized at pH of 13.80, showed a superior ability in syngas production with a H2/CO ratio of near 2 and also a proper stability against deactivation during the partial oxidation of methane.
-
Key words:
- partial oxidation /
- Co/γ-Al2O3 /
- nanoparticles /
- selectivity /
- pH value
-
Table 1 Composition of the prepared catalysts
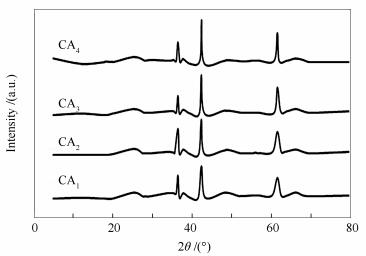
Sample NaOH concentration w/(mol·L-1) pH of synthesis solution CA1 0.01 11.92 CA2 0.02 12.47 CA3 0.1 13.04 CA4 0.6 13.80 Table 2 Textural parameters of the prepared catalysts
Sample Surface area A/(m2·g-1) Total pore volume v/(cm3·g-1) Average pore size d/nm Average CoO crystal size (XRD) d/nm Average CoO crystal size (TEM) d/nm γ-Al2O3 230 0.498 9.2 - - CA1 185.2 0.441 8.2 7.6 7.9 CA2 183.1 0.437 7.7 8.9 9.3 CA3 179.6 0.439 7.8 10.3 10.2 CA4 178.3 0.438 7.5 11.9 12.3 Table 3 Acid sites of prepared samples determined by NH3-TPD analysis
Sample NH3 uptake /(μmol·g-1) W M S CA1 72 139 127 CA2 83 155 99 CA3 89 161 87 CA4 95 180 71 weak (W), medium-strength (M) and strong (S) acid sites Table 4 H2-chemisorption and O2 pulse titration results for the synthesized catalysts
Sample Uncorrected dispersion /%a Uncorrected cobalt size d/nmb Reduction degree /%c Corrected dispersion /%d Corrected cobalt size d/nme CA1 7.81 12.29 71.6 10.91 8.82 CA2 7.57 12.67 79.7 9.49 10.11 CA3 7.32 13.12 83.2 8.79 10.92 CA4 6.57 14.59 91.8 7.15 13.42 a: uncorrected dispersion= 100×number of metallic cobalt atoms on the surface/total number of metallic cobalt atoms; b: uncorrected cobalt size were measured by H2-chemisorption using 96/uncorrected dispersion; c: measured by O2 pulse titration; d: corrected dispersion=100× (uncorrected dispersion/reduction degree); e: corrected cobalt size= (uncorrected cobalt size×reduction degree)/100 Table 5 Results of catalytic activity in the partial oxidation of methane
Catalyst Reaction temperature T/K xCH4 /% Selectivity s/% H2 yield w/%/CO ratio H2/CO ratio CO CO2 CA1 923 40.33 31.08 19.73 33.98 1.76 1023 51.23 41.57 13.58 52.71 1.8 CA2 923 46.24 36.79 17.42 39.67 1.82 1023 53.08 46.51 13.32 55.23 1.83 CA3 923 58.66 40.24 13.24 49.14 1.91 1023 65.09 48.19 10.23 60.59 1.88 CA4 923 62.67 55.24 8.28 61.17 1.98 1023 71.02 61.49 6.71 70.88 2.05 Table 6 Coke deposition and deactivation as a function of synthesis solution pH over the Co/Al2O3 catalysts
Sample Amount of coke formation /(mol×10-4) Deactivation /% CA1 2.423 66.1 CA2 1.987 51.23 CA3 1.744 38.46 CA4 1.214 27.15 -
[1] MOSAYEBI A, ABEDINI R. Partial oxidation of butane to syngas using nanostructure Ni/zeolite catalysts[J]. J Ind Eng Chem, 2014, 20(4):1542-1548. doi: 10.1016/j.jiec.2013.07.044 [2] MOSAYEBI A, ABEDINI R, BAKHSHI H. Ni@Pd nanoparticle with core-shell structure supported overγ-Al2O3 for partial oxidation process of methane to syngas[J]. Int J Hydrogen Energy, 2017, 42(30):18941-18950. doi: 10.1016/j.ijhydene.2017.06.027 [3] YU C L, HU J B, WENG W Z, ZHOU X C, CHEN X R. Preparation of Co/Ce0.5Zr0.5O2 catalysts and their catalytic performance in methane partial oxidation to produce synthesis gas[J]. J Fuel Chem Technol, 2012, 40(4):418-423. doi: 10.1016/S1872-5813(12)60019-X [4] YU C L, ZHOU X C, WENG W Z, HU J B, Chen X R, WEI L F. Effects of alkaline-earth strontium on the performance of Co/Al2O3 catalyst for methane partial oxidation[J]. J Fuel Chem Technol, 2012, 40(10):1222-1229. doi: 10.1016/S1872-5813(12)60123-6 [5] JAFARBEGLOO M, TARLANI A, WAHID MESBAH A, SAHEBDELFAR S. One-pot synthesis of NiO-MgO nanocatalysts for CO2 reforming of methane:The influence of active metal content on catalytic performance[J]. J Nat Gas Sci Eng, 2015, 27(2):1165-1173. https://www.sciencedirect.com/science/article/pii/S1875510015301980 [6] YU C, WENG W, SHU Q, MENG X, ZHANG B, CHEN X, ZHOU X. Additive effects of alkaline-earth metals and nickel on the performance of Co/γ-Al2O3 in methane catalytic partial oxidation[J]. J Nat Gas Chem, 2011, 20(2):135-139. doi: 10.1016/S1003-9953(10)60175-2 [7] NASABI M, LABBAFI M, MOUSAVI M E, MADADLOU A. Effect of salts and nonionic surfactants on thermal characteristics of egg white proteins[J]. Int J Biol Macromol, 2017, 102(1):970-976. https://www.sciencedirect.com/science/article/pii/S0141813017302192 [8] LARIMI A S, ALAVI S M. Ceria-zirconia supported Ni catalysts for partial oxidation of methane to synthesis gas[J]. Fuel, 2012, 102(1):366-371. https://www.sciencedirect.com/science/article/pii/S001623611200470X [9] KIM H W, KANG K M, KWAK H Y. Preparation of supported Ni catalysts with a core/shell structure and their catalytic tests of partial oxidation of methane[J]. Int J Hydrogen Energy, 2009, 34(8):3351-3359. doi: 10.1016/j.ijhydene.2009.02.036 [10] LI L, HE S, SONG S, ZHAO J, JI W, TAO C K. Fine-tunable Ni@porous silica core-shell nanocatalysts:Synthesis, characterization, and catalytic properties in partial oxidation of methane to syngas[J]. J Catal, 2012, 288(1):54-64. https://www.sciencedirect.com/science/article/pii/S0021951712000061 [11] XING C, AI P, ZHANG P, GAO X, YANG R, YAMANE N, SUN J, REUBROYCHAROEN P, TSUBAKI N. Fischer-Tropsch synthesis on impregnated cobalt-based catalysts:New insights into the effect of impregnation solutions and pH value[J]. J Energ Chem, 2016, 25(6):994-1000. doi: 10.1016/j.jechem.2016.09.008 [12] DELGADO J A, CASTILL S, CURULLA-FERR'E D, CLAVER C, GODARD C. Effect of pH on catalyst activity and selectivity in the aqueous Fischer-Tropsch synthesis catalyzed by cobalt nanoparticles[J]. Catal Commun, 2015, 71(1):88-92. https://www.sciencedirect.com/science/article/pii/S1566736715300376 [13] LI Z, SI M, LI X, LIU R, LIU R, LV J. Cobalt catalysts for Fischer-Tropsch synthesis:The effect of support, precipitant and pH value[J]. Chin J chem Eng, 2017. http://doi.org/10.1016/j.cjche.2017.11.001. doi: 10.1016/j.cjche.2017.11.001 [14] LIU J, YU J, SU F B, XU G W. Inter correlation of structure and performance of Ni-Mg/Al2O3 catalysts prepared with different methods for syngas methanation[J]. Catal Sci Technol, 2014, 4(2):472-481. doi: 10.1039/C3CY00601H [15] BAE J W, LEE Y J, PARK Y J, JUN K W. Influence of pH of the Impregnation solution on the catalytic properties of Co/Alumina for FTS[J]. Energy Fuels, 2008, 22(5):2885-2891. doi: 10.1021/ef800155v [16] MOSAYEBI A, HAGHTALAB A. The comprehensive kinetic modeling of the Fischer-Tropsch synthesis over Co@Ru/γ-Al2O3 core-shell structure catalyst[J]. Chem Eng J, 2015, 259(1):191-204. doi: 10.1021/cm0111074 [17] MOSAYEBI A, MEHRPOUYA M A, ABEDINI R. The development of new comprehensive kinetic modelingfor Fischer-Tropsch synthesis process over Co-Ru/Al2O3 nano-catalyst in a fixed-bed reactor[J]. Chem Eng J, 2016, 286(1):416-426. http://www.researchgate.net/publication/284084679_The_development_of_new_comprehensive_kinetic_modeling_for_Fischer-Tropsch_synthesis_process_over_Co-Ru-Al2O3_catalyst_in_a_fixed-bed_reactor [18] ABEDINI R, MOSAYEBI A, MOKHTARI M. Improved CO2 separation of azide cross-linked PMPmixed matrix membrane embedded by nano-CuBTC metal organic framework[J]. Process Saf Environ, 2018, 114(1):229-239. http://www.sciencedirect.com/science/article/pii/S0957582018300028 [19] HAGHTALAB A, MOSAYEBI A. Co@Ru nanoparticle with core-shell structure supported over Al2O3 for Fischer-Tropsch synthesis[J]. Int J Hydrogen Energy, 2014, 39(33):18882-18893. doi: 10.1016/j.ijhydene.2014.09.074 [20] KHODAKOV A Y, CHU W, FONGARLAND P. Recent advances in the liquid-phase syntheses of inorganic nanoparticles, Advances in the development of novel cobalt Fischer-Tropsch catalysts for synthesis of Long-Chain hydrocarbons and clean fuels[J]. Chem Rev, 2007, 107(5):1692-744. doi: 10.1021/cr050972v [21] PARK J Y, LEE Y J, KARANDIKAR P R, JUN K W, HA K S, PARK H. Fischer-Tropsch catalyst deposited with size controlled Co3O4 nanocrystals:effect of particle size on catalytic activity and stability[J]. Appl Catal A:Gen, 2012, 411-412(1):15-23. https://www.sciencedirect.com/science/article/pii/S0021951715000585 [22] MOSAYEBI A, ABEDINI R. Detailed kinetic study of Fischer-Tropsch synthesis for gasoline production over Co-Ni/HZSM-5 nano-structure catalyst[J]. Int J Hydrogen Energy, 2017, 42(44):27013-27023. doi: 10.1016/j.ijhydene.2017.09.060 [23] WANG S, YIN Q, GUO J, RU B, ZHU L. Improved Fischer-Tropsch synthesis for gasoline over Ru, Ni promoted Co/HZSM-5 catalysts[J]. Fuel, 2013, 108(1):597-603. https://www.sciencedirect.com/science/article/pii/S0016236113001099 [24] BAYRAKDAR E, GVRKAYNAKALTINÇ EKIÇT, ÖKSVZÖMER M A F. Effects of PVP on the preparation of nanosized Al2O3 supported Ni catalysts by polyol method for catalytic partial oxidation of methane[J]. Fuel Process Technol, 2013, 110(1):167175. http://www.sciencedirect.com/science/article/pii/S0378382012004560 [25] FERREIRA A C, FERRARIA A M, BOTEIHO A M, GONCALVE A P, CORREIA C. Partial oxidation of methane over bimetallic nickel-lanthanide oxides[J]. J Alloys Compd, 2010, 489(1):316-323. doi: 10.1016/j.jallcom.2009.09.082 [26] TAKENAKA S, ORITA Y, UMEBAYASHI H, MATSUNE H, KISHIDA M. High resistance to carbon deposition of silica-coated Ni catalysts in propane stream reforming[J]. Appl Catal A:Gen, 2008, 351(2):189-194. doi: 10.1016/j.apcata.2008.09.017 [27] RUCKENSTEIN E, WANG H Y. Carbon deposition and catalytic deactivation during CO2 reforming of CH4 over Co/Al2O3 catalysts[J]. J Catal, 2002, 205(2):289-293. doi: 10.1006/jcat.2001.3458 [28] LØDENG R, BJØRGUM E, ENGER B C, EILERTSEN J L, HOLMEN A, KROGH B, RØNNEKLEIV M, RYTTER E. Catalytic partial oxidation of CH4 to H2 over cobalt catalysts at moderate temperatures[J]. Appl Catal A:Gen, 2007, 333(1):11-23. doi: 10.1016/j.apcata.2007.08.038 -





 下载:
下载: