Kinetic study of the decomposition of methane over Ni-Mg composite catalyst for hydrogen production
-
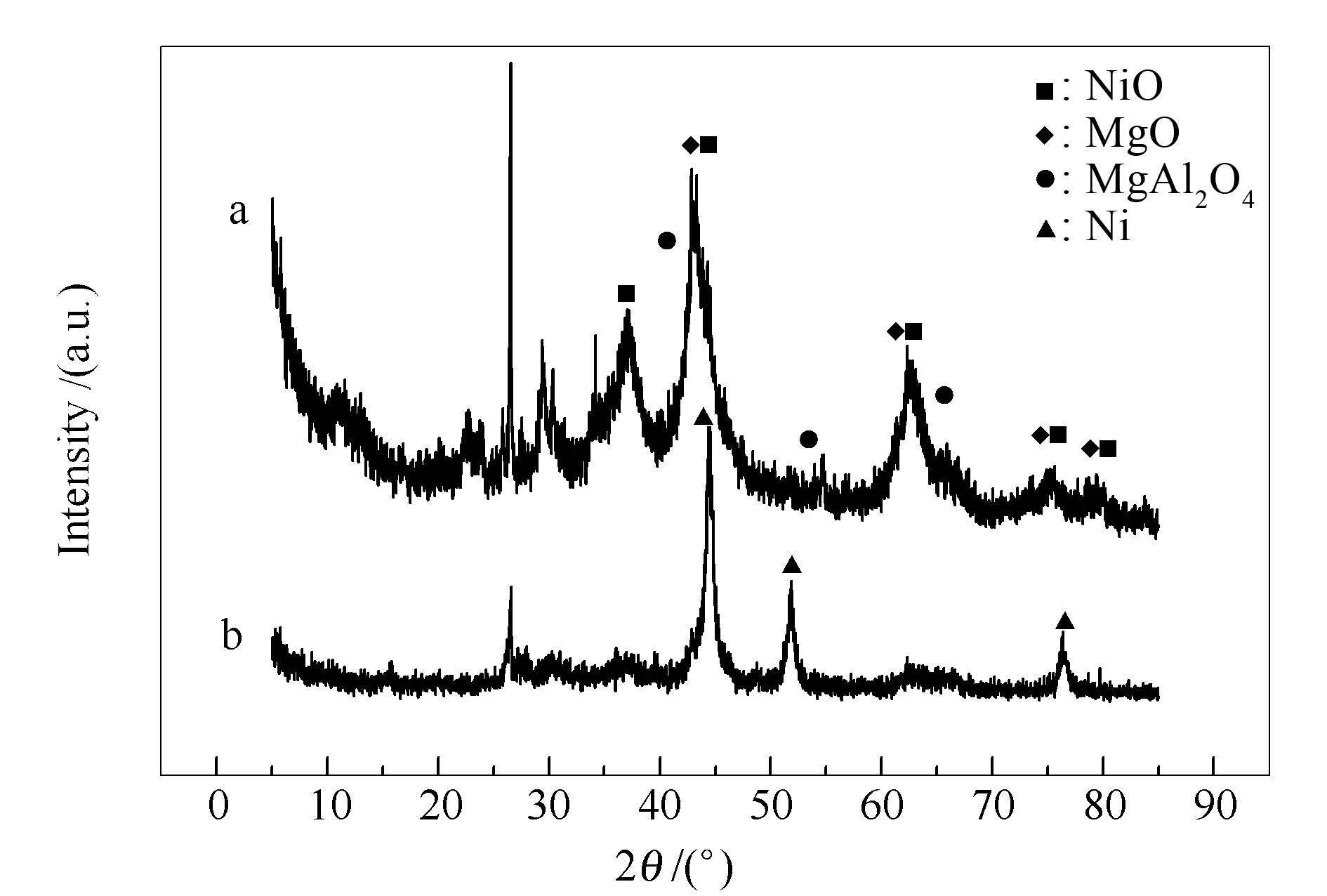
摘要: 基于定温热重实验,建立了甲烷催化裂解反应动力学模型和催化剂表面积炭失活动力学模型。其中,甲烷催化裂解动力学模型将初始产氢速率视为催化剂未积炭条件下的动力学基础数据;催化剂表面积炭失活动力学则基于甲烷催化裂解速率的降低。实验使用Ni-Mg复合催化剂,分别在535、585、635℃,甲烷分压104、2×104、3×104 Pa条件下展开甲烷催化裂解动力学特性研究。结果表明,甲烷催化裂解的反应级数为0.5,活化能为82 kJ/mol;Ni-Mg复合催化剂反应失活级数为0.5,催化剂失活活化能为118 kJ/mol。实验条件下均制得了多壁碳纳米管。
-
关键词:
- 甲烷催化裂解 /
- 制氢 /
- Ni-Mg复合催化剂 /
- 动力学
Abstract: The kinetic and deactivation kinetic models of methane catalytic cracking were established based on the data of thermal gravimetric analyzer.The kinetic model of methane catalytic cracking was established by the data of initial hydrogen production rate under the condition of no carbon deposition.The deactivation kinetic model was established by the reduced rate of methane catalytic cracking.The experiment was carried out over Ni-Mg composite catalyst, at the temperature of 535, 585, 635℃ and the methane partial pressures were 104, 2×104, 3×104 Pa.The result shows that the reaction order and activation energy were 0.5 and 82 kJ/mol, the deactivation order and activation energy were 0.5 and 118 kJ/mol respectively.The multi-walled carbon nanotubes were all produced under the experimental conditions. -
表 1 实验工况表
Table 1 Experimental conditions
Run Temperature t/℃ Methane partial pressure p/Pa 1 535 104 2 535 2×104 3 535 3×104 4 585 104 5 585 2×104 6 585 3×104 7 635 104 8 635 2×104 9 635 3×104 -
[1] 中国科学院能源领域战略研究组. 中国2050年能源科技发展路线图[C]. 北京:科学出版社, 2009.Research group on energy strategy of chinese academy of sciences. China energy science and technology development roadmap in 2050[C]. Beijing:Science Press, 2009. [2] 2050 中国能源和碳排放研究课题组. 2050中国能源和碳排放报告[C]. 北京:科学出版社, 2009.2050 research group on energy and carbon emissions in China. 2050 China energy and carbon emissions report[C]. Beijing:Science Press, 2009. [3] 张志, 陆光达, 唐涛, 秦城, 黄火根, 郑少涛, 宋江峰. Ni/氧化金刚石催化裂解甲烷制氢技术研究[J]. 材料导报, 2007, 21(S1):270-273.ZHANG Zhi, LU Guang-da, TANG Tao, QIN Cheng, HUANG Huo-gen, ZHENG Shao-tao, SONG Jiang-feng. Catalytic decompositon of methane by Ni/oxidized diamond[J]. Mater Rev, 2007, 21(S1):270-273. [4] 靳立军, 王焦飞, 郑宇, 胡浩权. 炭催化甲烷裂解制氢研究进展[J]. 化工进展, 2014, 33(12):3125-3132.JIN Li-jun, WANG Jiao-fei, ZHENG Yu, HU Hao-quan. Research progress of hydrogen production by catalytic decomposition of methane over carbon catalysts[J]. Chem Ind Eng Prog, 2014, 33(12):3125-3132. [5] 潘智勇, 沈师孔. Ni/SiO2催化剂上甲烷催化裂解制氢[J]. 燃料化学学报, 2003, 31(5):466-470.PAN Zhi-yong, SHEN Shi-kong. The decompositon of methane in hydrogen production over Ni/SiO2 catalyst[J]. J Fuel Chem Technol, 2004, 31(5):466-470. [6] LEE M B, YANG Q Y, TANG S L, CEYER S T. Activated dissociative chemisorption of CH4 on Ni (111):Observation of a methyl radical and implication for the pressure gap in catalysis[J]. J Chem Phys, 1986, 85(3):1693-1694. doi: 10.1063/1.451211 [7] RODRIGUEZ N M. A review of catalytically grown carbon nanofibers[J]. J Mater Res, 1993, 8(12):3233-3250. doi: 10.1557/JMR.1993.3233 [8] 王毅, 周金梅, 李清彪, 林国栋, 张鸿斌. CH4在Ni-Mg-O催化剂上裂解生长碳纳米管的本征动力学[J]. 厦门大学学报(自然科学版), 2004, 43(4):522-526.WANG Yi, ZHOU Jin-mei, LI Qing-biao, LIN Guo-dong, ZHANG Hong-bin. Kinetic studies of the decompositon of methane for growth of carbon nanotubes over Ni-Mg-O catalyst[J]. J Xiamen Univ(Nat Sci), 2004, 43(4):522-526. [9] MURADOV N. Hydrogen via methane decomposition:An application for decarbonization of fossil fuels[J]. Int J Hydrogen Energy, 2001, 26(11):1165-1175. doi: 10.1016/S0360-3199(01)00073-8 [10] MURADOV N. Catalysis of methane decomposition over elemental carbon[J]. Catal Commun, 2001, 2(3):89-94. https://www.researchgate.net/publication/222971069_Catalysis_of_methane_decomposition_over_elemental_carbon [11] MURADOV N. Thermocatalytic CO2-free production of hydrogen from hydrocarbon fuels[C]. Proceedings of the 2000 Hydrogen Program Review, NREL/CP-570-28890. 2000. [12] KIM M H, LEE E K, JUN J H, KONG S J, HAN G Y, LEE B K, LEE T, YOON K J. Hydrogen production by catalytic decomposition of methane over activated carbons:Kinetic study[J]. Int J Hydrogen Energy, 2004, 29(2):187-193. doi: 10.1016/S0360-3199(03)00111-3 [13] SHILAPURAM V, OZALP N, OSCHATZ M, BORCHARDT L, KASKEL S, LACHANCE R. Thermogravimetric analysis of activated carbons, ordered mesoporous carbide-derived carbons, and their deactivation kinetics of catalytic methane decomposition[J]. Ind Eng Chem Res, 2013, 53(5):1741-1753. [14] 罗渝然, 俞书勤, 张祖德, 姚天扬, 高盘良. 再谈什么是活化能——Arrhenius活化能的定义、解释、以及容易混淆的物理量[J]. 大学化学, 2010, 25(3):35-42.LUO Yu-ran, YU Shu-qin, ZHANG Zu-de, YAO Tian-yang, GAO Pan-liang. What is the activation energy-the definition, explanation, and the physical of the activation energy[J]. Univ Chem, 2010, 25(3):35-42. [15] HOLMEN A, ROKSTAD O A, SOLBAKKEN A. High-temperature pyrolysis of hydrocarbons. 1. Methane to acetylene[J]. Ind Eng Chem Process Des Dev, 1976, 15(3):439-444. doi: 10.1021/i260059a017 [16] DAHL J K, BAROCAS V H, CLOUGH D E, WEIMER A W. Intrinsic kinetics for rapid decomposition of methane in an aerosol flow reactor[J]. Int J Hydrogen Energy, 2002, 27(4):377-386. doi: 10.1016/S0360-3199(01)00140-9 [17] ABBAS H F, WAN DAUD W M A. Thermocatalytic decomposition of methane using palm shell based activated carbon:kinetic and deactivation studies[J]. Fuel Process Technol, 2009, 90(9):1167-1174. doi: 10.1016/j.fuproc.2009.05.024 [18] FU X, CUI X, WEI X, MA J. Investigation of low and mild temperature for synthesis of high quality carbon nanotubes by chemical vapor deposition[J]. Appl Surf Sci, 2014, 292:645-649. doi: 10.1016/j.apsusc.2013.12.026 [19] MAJEWSKA J, MICHALKIEWICZ B. Carbon nanomaterials produced by the catalytic decomposition of methane over Ni/ZSM-5:Significance of Ni content and temperature[J]. New Carbon Mater, 2014, 29(2):102-108. doi: 10.1016/S1872-5805(14)60129-3 [20] SIMON A, SEYRING M, KÄMNITZ S, RICHTER H, VOIGT I RETTENMAYR M. Carbon nanotubes and carbon nanofibers fabricated on tubular porous Al2O3 substrates[J]. Carbon, 2015, 90:25-33. doi: 10.1016/j.carbon.2015.03.048 -





 下载:
下载: