Experimental study on selective catalytic reduction of NO by C3H6 over Fe/Ti-PILC catalysts
-
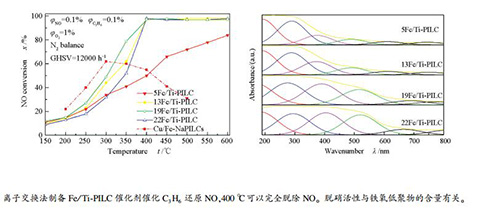
摘要: 采用二氧化钛对蒙脱土进行柱撑改性后,以离子交换法制备了铁负载二氧化钛柱撑蒙脱土催化剂Fe/Ti-PILC,考察了其在富氧条件下催化C3H6选择性还原NO(C3H6-SCR)的性能。并借助N2等温吸附-脱附、XRD、UV-vis、H2-TPR、Py-FTIR等表征方法研究了催化剂的结构与性能之间的关系。结果表明,所制备的19Fe/Ti-PILC催化剂在400 ℃时即可实现到NO的完全脱除,N2选择性能够达到90%以上。且具有较好的抗水蒸气和抗SO2的能力。N2吸附-脱附和XRD结果显示,蒙脱土的结构被撑开,交联柱撑有效,形成了较大的比表面积和孔体积。UV-vis结果表明,催化剂的脱硝活性与铁氧低聚物种FexOy的含量有关,Py-FTIR结果表明,催化剂表面同时存在Lewis酸和Brønsted酸,Fe3+负载到柱撑黏土中能够显著增加Lewis酸的含量,Lewis酸是影响催化剂脱硝活性的影响因素之一。H2-TPR表征表明,催化剂在400 ℃左右有较强的还原能力,催化剂的还原能力主要体现为Fe3+→Fe2+的还原。Abstract: Ti-pillared interlayer clay (PILC)-based catalysts ion exchanged with Fe were prepared and used for selective catalytic reduction of NOx using propylene as the reducing agent under oxygen-rich conditions. The relationship between structure and properties of the catalysts was studied using N2-adsorption/desorption, XRD, UV-vis, H2-TPR, and Py-FTIR. The results show that the prepared 19Fe/Ti-PILC catalyst can achieve complete removal of NO at 400 ℃, and N2 selectivity can reach over 90% and has better resistance to water vapor and SO2. N2-isothermal adsorption/desorption and XRD results show that structure of montmorillonite is opened, cross-linked pillars are effective, and a large specific surface area and pore volume are formed. UV-vis results show that the denitrification activity of the catalyst is related to content of oligomeric FexOy. Py-FTIR results show that both Lewis acid and Brønsted acid are presented on the catalyst surface. Fe3+ loading into the pillared clay can significantly increase the Lewis acid content. Lewis acid is one of the influencing factors on the denitrification activity of the catalyst. H2-TPR indicates that the catalyst has a strong reduction ability at about 400 ℃, and the reduction ability of the catalyst is mainly represented by the reduction of Fe3+→Fe2+.
-
Key words:
- oxygen-rich conditions /
- pillared clay /
- selective catalytic reduction /
- propylene
-
表 1 不同负载量的催化剂的织构特征
Table 1 Properties of catalysts with different loadings of Fe
Catalyst Fe w/(mg·g-1) ABET/(m2·g-1) Pore volume v/(cm3·g-1) Pore diameter d/nm Original clay - 24 0.099 16.44 Ti-PILC - 203 0.240 4.73 13Fe/Ti-PILC 135.2 166 0.207 4.97 19Fe/Ti-PILC 192.1 190 0.222 5.07 22Fe/Ti-PILC 221.0 179 0.209 4.73 表 2 通过UV-vis光谱的定量分析确定的子带面积百分比
Table 2 Percentage of the area of the sub-bands by quantitative analysis of the UV-vis spectra
Catalyst Percentage /% I1 I2 I3 5Fe/Ti-PILC 70 14 16 13Fe/Ti-PILC 56 23 21 19Fe/Ti-PILC 41 29 30 22Fe/Ti-PILC 39 24 37 I1, I2, and I3 represent isolated Fe3+ species, small FexOy oligomers, and Fe2O3 particles species, respectively 表 3 不同负载量的催化剂中Brønsted酸和Lewis酸的含量
Table 3 Brønsted and Lewis acid content of different catalysts
Sample 40 ℃ desorption /(μmol·g-1) 170 ℃ desorption /(μmol·g-1) 300 ℃ desorption /(μmol·g-1) B L B L B L Ti-PILC 9.99 382.61 4.05 127.14 2.78 83.83 5Fe/Ti-PILC 46.84 398.59 31.06 197.24 19.36 133.96 13Fe/Ti-PILC 13.96 636.18 10.34 189.85 5.17 122.80 19Fe/Ti-PILC 7.17 688.40 37.83 311.51 24.94 234.59 -
[1] IWAMOTO M, YAHIRO H, YU U Y. Selective reduction of NO by lower hydrocarbons in the presence of O2 and SO2 over copper ion-exchanged zeolites[J]. Catal, 1990, 32(6):430-433. http://cn.bing.com/academic/profile?id=cf5d3c3705d15b224ce43f4bb7a701ab&encoded=0&v=paper_preview&mkt=zh-cn [2] HELD W, KOENIG A, RICHTER T, PUPPE L. Catalytic NOx reduction in net oxidizing exhaust gas[J]. SAE Trans, 1990, 99(4):209-216. http://cn.bing.com/academic/profile?id=9ad9c1fb075cb3e4cc9efcb308c9deef&encoded=0&v=paper_preview&mkt=zh-cn [3] 周皞, 苏亚欣, 邓文义, 钟方川.金属氧化物类催化剂上HC-SCR研究进展[J].环境科学与技术, 2016, 39(1):93-100. http://www.cnki.com.cn/Article/CJFDTOTAL-FJKS201601015.htmZHOUs Hao, SU Ya-xin, DENG Wen-yi, ZHONG Fang-chuan. A review of HC-SCR over metal oxides-based catalysts[J]. Environ Sci Technol, 2016, 39(1):93-100. http://www.cnki.com.cn/Article/CJFDTOTAL-FJKS201601015.htm [4] 王琪莹, 文焱炳, 董新法, 林维明.交联粘土合成及其在C3H6选择性催化还原NOx中的应用研究[J].高校化学工程学报, 2006, 20(4):598-603. doi: 10.3321/j.issn:1003-9015.2006.04.019WANG Qi-yin, WEN Yan-bing, DONG Xin-fa, LIN Wei-ming. Crosslinked clay synthesis and its application in selective catalytic reduction of NOx with C3H6[J]. J Chem Eng Chin Univ, 2006, 20(4):598-603. doi: 10.3321/j.issn:1003-9015.2006.04.019 [5] YASHNIK S A, SALNIKOV A V, VASENIN N T, ANUFRIENKO V F, ISMAGILOV Z R. Regulation of the copper-oxide cluster structure and DeNOx activity of Cu-ZSM-5 catalysts by variation of OH/Cu2+[J]. Catal Today, 2012, 197(1):214-227. doi: 10.1016/j.cattod.2012.08.033 [6] KUMAR P A, REDDY M P, JU LK, HYUN-SOOK B, PHIL H H. Low temperature propylene SCR of NO by copper alumina catalyst[J]. J Mol Catal A:Chem, 2008, 291(1/2):66-74. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=JJ028888039 [7] LONG R Q, CHANG M T, YANG R T. Enhancement of activities by sulfation on Fe-exchanged TiO2-pillared clay for selective catalytic reduction of NO by ammonia[J]. Appl Catal B:Environ, 2001, 33(2):97-107. doi: 10.1016/S0926-3373(01)00173-4 [8] VAUGHAN D E W, LUSSIER R J, MAGEE J S. Pillared interlayered clay materials useful as catalysts and sorbents: CA, US4176090[P]. 1979. [9] YANG R T, THARAPPIWATTANANON N, LONG R Q. Ion-exchanged pillared clays for selective catalytic reduction of NO by ethylene in the presence of oxygen[J]. Appl Catal B:Environ, 1998, 19(3/4):289-304. http://cn.bing.com/academic/profile?id=0430e23435424ea821024df65f7495d5&encoded=0&v=paper_preview&mkt=zh-cn [10] VALVERDE J L, LUCAS A D, SÁNCHEZ P, DORADO F, ROMERO A. Cation exchanged and impregnated ti-pillared clays for selective catalytic reduction of NOx by propylene[J]. Appl Catal B:Environ, 2003, 43(1):43-56. doi: 10.1016/S0926-3373(02)00274-6 [11] LU G, LI X Y, QU Z P, ZHAO Q D, ZHAO L, CHEN G H. Copper-ion exchanged Ti-pillared clays for selective catalytic reduction of NO by propylene[J]. Chem Eng J, 2011, 168(3):1128-1133. doi: 10.1016/j.cej.2011.01.095 [12] 窦逸峰, 苏亚欣, 陆哲惺, 周皞, 邓文义.乙烷在金属铁表面还原NO的实验研究[J].燃料化学学报, 2015, 43(10):1273-1280. doi: 10.3969/j.issn.0253-2409.2015.10.017DOU Yi-feng, SU Ya-xin, LU Zhe-xing, ZHOU Hao, DENG Wen-yi. Experimental study of NO reduction by ethane over iron[J]. J Fuel Chem Technol, 2015, 43(10):1273-1280. doi: 10.3969/j.issn.0253-2409.2015.10.017 [13] 梁俊青, 苏亚欣, 周皞, 邓文义.金属铁与丙烯共同还原NO的特性与机理[J].燃料化学学报, 2016, 44(8):977-984. doi: 10.3969/j.issn.0253-2409.2016.08.011LIANG Jun-qing, SU Ya-xin, ZHOU Hao, DENG Wen-yi. Performance and mechanism of NO reduction by iron combined with propene[J]. J Fuel Chem Technol, 2016, 44(8):977-984. doi: 10.3969/j.issn.0253-2409.2016.08.011 [14] 苏亚欣, 苏阿龙, 任立铭, 邓文义. SO2对甲烷在金属铁表面还原NO的反应影响[J].燃料化学学报, 2014, 42(3):377-384. http://d.old.wanfangdata.com.cn/Periodical/rlhxxb201403019SU Ya-xin, SU A-long, REN Li-ming, DENG Wen-yi. Effect of SO2 on NO reduction by methane over iron[J]. J Fuel Chem Technol, 2014, 42(3):377-384. http://d.old.wanfangdata.com.cn/Periodical/rlhxxb201403019 [15] 周皞, 苏亚欣, 戚越舟, 陆哲惺, 邓文义.水蒸气对甲烷在金属铁表面还原NO行为的影响[J].燃料化学学报, 2014, 42(11):1378-1386. doi: 10.3969/j.issn.0253-2409.2014.11.016ZHOU Hao, SU Ya-xin, QI Yue-zhou, LU Zhe-xing, DENG Wen-yi. Effect of water vapor on NO reduction by methane over iron[J]. J Fuel Chem Technol, 2014, 42(11):1378-1386. doi: 10.3969/j.issn.0253-2409.2014.11.016 [16] 钱文燕, 苏亚欣, 杨溪, 袁旻昊, 邓文义, 赵兵涛. Fe/Al-PILC催化C3H6选择性还原NO的实验研究[J].燃料化学学报, 2017, 45(12):1499-1507. doi: 10.3969/j.issn.0253-2409.2017.12.012QIAN Wen-yan, SU Ya-xin, YANG Xi, YUAN Min-hao, DENG Wen-yi, ZHAO Bing-tao. Experimental study on selective catalytic reduction of NO with propene over iron based catalysts supported on aluminum pillared clays[J]. J Fuel Chem Technol, 2017, 45(12):1499-1507. doi: 10.3969/j.issn.0253-2409.2017.12.012 [17] 叶青, 闫立娜, 霍飞飞, 王海平, 程水源, 康天放. Cu负载Fe柱撑钠化海泡石:结构特点及其丙烯选择性催化还原NO性质研究[J].化学学报, 2011, 69(13):1524-1532. http://d.old.wanfangdata.com.cn/Periodical/hxxb201113005YE Qing, YAN Li-na, HUO Fei-fei, WANG Hai-ping, CHENG Shui-yuan, KANG Tian-fang. Cu-supportedon Fe-pillared sepiolite:Characterization and selective catalytic reduction(SCR)of NO by propene[J]. Acta Chim Sin, 2011, 69(13):1524-1532. http://d.old.wanfangdata.com.cn/Periodical/hxxb201113005 [18] YANG T T, BI H T, CHENG X. Effects of O2, CO2 and H2O on NOx adsorption and selective catalytic red uction over Fe/ZSM-5[J]. Appl Catal B:Environ, 2011, 102(1/2):163-171. [19] MARTÍNEZ-HERNÁNDEZ A, FUENTES G A. Redistribution of cobalt species in Co-ZSM-5 during operation under wet conditions in the reduction of NOx by propane[J]. Appl Catal B:Environ, 2005, 57(3):167-174. doi: 10.1016/j.apcatb.2004.10.018 [20] KOMVOKIS V G, ILIOPOULOU E F, VASALOS I A, TRIANTAFYLLIDIS K S, MARSHALL C L. Development of optimized Cu-ZSM-5 deNOx catalytic materials both for HC-SCR applications and as FCC catalytic additives[J]. Appl Catal A:Environ, 2007, 325(2):345-352. doi: 10.1016/j.apcata.2007.02.035 [21] KIM B S, LEE S H, PARK Y T, HAM S W, CHAE H J, NAM I S. Selective catalytic reduction of NOx, by propene over copper-exchanged pillared clays[J]. Korean J Chem Eng, 2001, 18(5):704-710. doi: 10.1007/BF02706390 [22] LONG R Q, YANG R T. Selective catalytic reduction of NO with ammonia over V2O5doped TiO2 pillared clay catalysts[J]. Appl Catal B:Environ, 2000, 24(1):13-21. doi: 10.1016/S0926-3373(99)00092-2 [23] GREGG S J, SING K S W. Adsorption, Surface Area and Porosity[M]. New York:Academic Press Inc, 1982. [24] LONG R Q, YANG R T. Selective catalytic reduction of nitrogen oxides by ammonia over Fe3+-exchanged TiO2-pillared clay catalysts[J]. J Catal, 1999, 186(2):254-268. doi: 10.1006/jcat.1999.2558 [25] CHMIELARZ L, PIWOWARSKA Z, KUŚTROWSKI P, WEGRZYN A, GIL B, KOWALCZYK A, DUDEK B, DZIEMBAJ R, MICHALIK M. Comparison study of titania pillared interlayered clays and porous clay heterostructures modified with copper and iron as catalysts of the DeNOx process[J]. Appl Clay Sci, 2011, 53(2):164-173. doi: 10.1016/j.clay.2010.12.009 [26] 沈伯雄, 马宏卿, 杨晓燕, 姚燕. Mn-CeOx/Ti-PILC的制备、表征及脱硝性能研究[J].燃料化学学报, 2012, 40(5):615-620. doi: 10.3969/j.issn.0253-2409.2012.05.017SHEN Bo-xiong, MA Hong-qing, YANG Xiao-yan, YAO Yan. Study on preparation, characterization and de-NO activity of Mn-CeOx/Ti-PILC[J]. J Fuel Chem Technol, 2012, 40(5):615-620. doi: 10.3969/j.issn.0253-2409.2012.05.017 [27] OLIVEIRA L C A, RIOS R V R A, FABRIS J D, SAPAG K, GARG V K, LAGO R M. Clay-iron oxide magnetic composites for the adsorption of contaminants in water[J]. Appl Clay Sci, 2003, 22(4):169-177. doi: 10.1016/S0169-1317(02)00156-4 [28] KUMAR M S, SCHWIDDER M, GRÜNERT W, BRUCKNER A. On the nature of different iron sites and their catalytic role in Fe-ZSM-5 DeNOx, catalysts:New insights by a combined EPR and UV/VIS spectroscopic approach[J]. J Catal, 2004, 227(2):384-397. doi: 10.1016/j.jcat.2004.08.003 [29] BRANDENBERGER S, KRÖCHER O, WOKAUN A, TISSLER A, ALTHOFF R. The role of Brønsted acidity in the selective catalytic reduction of NO with ammonia over Fe-ZSM-5[J]. J Catal, 2009, 268(2):297-306. doi: 10.1016/j.jcat.2009.09.028 [30] DATKA J, TUREK A M, JEHNG J M, WACHS I E. Acidic properties of supported niobium oxide catalysts:An infrared spectroscopy investigation[J]. J Catal, 1992, 135(135):186-199. doi: 10.1016-0021-9517(92)90279-Q/ [31] SULTANA A, HANEDA M, FUJITANI T, HAMADA H. Influence of Al2O3 support on the activity of Ag/Al2O3 catalysts for SCR of NO with decane[J]. Catal Lett, 2007, 114(1):96-102. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=JJ025335851 [32] CHMIELARZ L, PIWOWARSKA Z, KUŚTROWSKIP, WEGRZYN A, GIL B, KOWALCZYK A, DUDEK B, DZIEMBAJ R, MICHALIK M. Comparison study of titania pillared interlayered clays and porous clay heterostructures modified with copper and iron as catalysts of the DeNOx process[J]. Appl Clay Sci, 2011, 53(2):164-173. doi: 10.1016/j.clay.2010.12.009 [33] TOLEDO-ANTONIO J A, CORTÉS-JÁCOME M A, NAVARRETE J, ANGELES-CHAVEZ C, LOPEZ-SALINAS E, RENDON-RIVERA A. Morphology induced CO, pyridine and lutidine adsorption sites on TiO2:Nanoparticles, nanotubes and nanofibers[J]. Catal Today, 2010, 155(3/4):247-254. http://cn.bing.com/academic/profile?id=ebfdce3c1a2840fc3ac70c1c9e514c9e&encoded=0&v=paper_preview&mkt=zh-cn -





 下载:
下载: