Influence of olefin on the mechanism of thiophene adsorption on the active species of Al-MCM-41 mesoporous zeolites
-
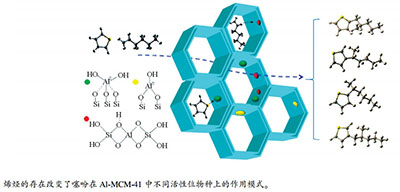
摘要: 采用后嫁接法制备了不同铝负载量的Al-MCM-41分子筛。运用XRD、N2吸附-脱附、NH3-TPD、Py-FTIR等方法对分子筛进行物性表征,利用固定床评价其对噻吩的吸附性能。通过将分子筛吸附噻吩能力与分子筛的酸性质及织构性质进行关联,考察烯烃存在对Al-MCM-41活性位物种吸附脱硫机制的影响。结果表明,铝物种的引入即产生了B酸中心,也同时产生了两种类型的L酸中心L1和L2。引入低含量铝物种利于形成B酸中心和L1型酸中心,引入高含量铝物种利于形成L2型酸中心。其中,L2型酸中心对噻吩的吸附效果最佳。烯烃和噻吩在B酸中心发生竞争吸附和催化转化反应,且催化转化反应占主导地位。L2酸中心的存在促进了B酸中心上的催化转化反应,其所生成的大分子硫化物取代噻吩吸附在分子筛酸活性中心上提高了Al-MCM-41分子筛的饱和吸附硫容量。
-
关键词:
- Al-MCM-41分子筛 /
- 噻吩 /
- 烯烃 /
- 酸性中心 /
- 吸附脱硫机制
Abstract: The Al-MCM-41 zeolites with different Al contents were prepared by post-grafting method and characterized by means of XRD, N2 adsorption-desorption, NH3-TPD, and Py-FTIR. The adsorptive performance of thiophene on these samples was investigated in a fixed bed by using micro coulombmeter and GC-SCD technique. The thiophene adsorption capacity was correlated with the acid properties and texture properties of the molecular sieve, and the effect of olefin on the adsorption desulfurization mechanism of active species in Al-MCM-41 was investigated. The results show that the introduction of lower content aluminum species is conducive to the formation of B (Brønsted) acid center and L1 (Lewis) acid center, while higher content aluminum species is conducive to the formation of L2 (Lewis) acid center. L2 acid center exhibits a far stronger thiophene adsorption ability than L1 acid center that has a slightly stronger thiophene adsorption ability than B acid center. Competitive adsorption and catalytic conversion of olefin and thiophene take place on the B acid center, and the catalytic reaction is dominated. The existence of L2 acid center greatly promotes the catalytic conversion reaction on the B acid center. The adsorption of macromolecular sulfide instead of thiophene increases the saturated adsorption capacity of Al-MCM-41 zeolites.-
Key words:
- Al-MCM-41 zeolite /
- thiophene /
- olefin /
- acid active center /
- adsorption desulfurization mechanism
-
图 2 不同铝含量Al-MCM-41样品的N2吸附-脱附等温线和孔径分布
Figure 2 Nitrogen adsorption-desorption isotherms and BJH pore size distributions of the Al-MCM-41 samples
◆: Al-MCM-41(∞) adsorption; ◇: Al-MCM-41(∞) desorption; ▼: Al-MCM-41(60) adsorption; ▽: Al-MCM-41(60)desorption; ★: Al-MCM-41(30)adsorption; ☆: Al-MCM-41(30)desorption; ●: Al-MCM-41(10) adsorption; ○: Al-MCM-41(10)desorption
表 1 不同硅铝比Al-MCM-41分子筛的结构参数
Table 1 Textural parameters of the different Si/Al ratio Al-MCM-41 samples
Sample ABET
/(m2·g-1)Amic
/(m2·g-1)Ameso
/(m2·g-1)vp
/(cm3·g-1)Average pore
diameter d/nma0/nm w/%
(Al)Al-MCM-41(∞) 1069.3 12.3 1057 1.0 2.79 3.22 - Al-MCM-41(60) 1067.4 12.5 1054.9 0.9 2.82 3.26 0.70 Al-MCM-41(30) 1063.1 12.8 1050.3 1.0 2.83 3.27 1.41 Al-MCM-41(10) 1046 9.6 1036.4 0.85 2.70 3.12 3.99 w/% (Al):actual Al content in samples which is tested by ICP-MS 表 2 不同铝含量Al-MCM-41样品中不同活性中心相对比例
Table 2 Proportion of active sites in Al-MCM-41 samples
Sample B/(B+L1+L2) L1/(B+L1+L2) L2/(B+L1+L2) Al-MCM-41(∞)(150 ℃) 0.41 0.53 0.06 Al-MCM-41(60)(150 ℃) 0.38 0.62 0 Al-MCM-41(30)(150 ℃) 0.53 0.47 0 Al-MCM-41(10)(150 ℃) 0.16 0.27 0.57 Al-MCM-41(∞)(400 ℃) 0.26 0.74 0 Al-MCM-41(60)(400 ℃) 0.27 0.73 0 Al-MCM-41(30)(400 ℃) 0.26 0.74 0 Al-MCM-41(10)(400 ℃) 0.16 0.30 0.54 note: proportion of active sites are calculated from the peak area of Py-FTIR spectra by emeis’s method[25] -
[1] 毛艳红, 刘冬梅, 王海彦, 王钰佳.碱酸改性ZSM-5分子筛催化剂的噻吩烷基化反应性能研究[J].燃料化学学报, 2017, 45(12):1456-1466. doi: 10.3969/j.issn.0253-2409.2017.12.007MAO Yan-hong, LIU Dong-mei, WANG Hai-yan, WANG Yu-jia. Study on the performance of alkali acid modified ZSM-5 catalysts for thiophene alkylation reaction[J]. J Fuel Chem Technol, 2017, 45(12):1456-1466. doi: 10.3969/j.issn.0253-2409.2017.12.007 [2] DEHGHAN R, ANBIA M. Zeolites for adsorptive desulfurization from fuels:A review[J]. Fuel Process Technol, 2017, 167:99-116. doi: 10.1016/j.fuproc.2017.06.015 [3] SONG C S. An overview of new approaches to deep desulfurization for ultra-clean gasoline, diesel fuel and jet fuel[J]. Catal Today, 2003, 86(1):211-263. doi: 10.1016-S0920-5861(03)00412-7/ [4] ZHOU A N, MA X L, SONG C S. Effects of oxidative modification of carbon surface on the adsorption of sulfur compounds in diesel fuel[J]. Appl Catal B:Environ, 2009, 87:190-199. doi: 10.1016/j.apcatb.2008.09.024 [5] 王洪国, 姜恒, 徐静, 孙兆林, 张晓彤, 朱赫礼, 宋丽娟.苯和1-辛烯对Ce(Ⅳ)Y分子筛选择性吸附脱硫的影响[J].物理化学学报, 2008, 24(9):1714-1718. doi: 10.3866/PKU.WHXB20080933WANG Hong-guo, JIANG Heng, XU Jing, SUN Zhao-lin, ZHANG Xiao-tong, ZHU He-li, SONG Li-juan. Effects of benzene and 1-octene on desulfurization by selective adsorption with Ce(Ⅳ)Y[J]. Acta Phys-Chim Sin, 2008, 24(9):1714-1718. doi: 10.3866/PKU.WHXB20080933 [6] DUAN L H, GAO X H, MENG X H, ZHANG H T, WANG Q, QIN Y C, ZHANG X T, SONG L J. Adsorption, co-adsorption, and reactions of sulfur compounds, aromatics, olefins over Ce-exchanged Y Zeolite[J]. J Phys Chem C, 2012, 116(49):25758-25756. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=798e75c99c165438968a1b98cd89a66e [7] 宋丽娟, 胡月婷, 秦玉才, 于文广, 张晓彤. NiY分子筛表面酸性影响其选择性吸附脱硫性能的机理研究[J].燃料化学学报, 2016, 44(9):1082-1088. doi: 10.3969/j.issn.0253-2409.2016.09.008SONG Li-juan, HU Yue-ting, QIN Yu-cai, YU Wen-guang, ZHANG Xiao-tong. Mechanism of effects of surface acidity on performance of adsorption desulfurization of NiY zeolites[J]. J Fuel Chem Technol, 2016, 44(9):1082-1088. doi: 10.3969/j.issn.0253-2409.2016.09.008 [8] ZU Y, QIN Y C, GAO X H, LIU H H, ZHANG X T, ZHANG J D, SONG L J. Insight into the correlation between the adsorption-transformation behaviors of methylthiophenes and the active sites of zeolites Y[J]. Appl Catal B:Environ, 2017, 203:96-107. doi: 10.1016/j.apcatb.2016.10.008 [9] 王旺银, 潘明雪, 秦玉才, 王凌涛, 宋丽娟. Cu(Ⅰ)Y分子筛表面酸性对其吸附脱硫性能的影响[J].物理化学学报, 2011, 27(5):1176-1180. doi: 10.3866/PKU.WHXB20110442WANG Wang-yin, PAN Ming-xue, QIN Yu-cai, WANG Ling-tao, SONG Li-juan. Effects of surface acidity on the adsorption desulfurization of Cu(Ⅰ)Y zeolites[J]. Acta Phys-Chim Sin, 2011, 27(5):1176-1180. doi: 10.3866/PKU.WHXB20110442 [10] SCHALLMOSER S, HALLER G L, SANCHEZ-SANCHEZ M, LERCHER J A. Role of spatial constraints of Brønsted acid sites for adsorption and surface reactions of linear pentenes[J]. J Am Chem Soc, 2017, 139(25):8646-8652. doi: 10.1021/jacs.7b03690 [11] RICHARDEAU D, JOLY G, CANAFF C, MAGNOUX P, GUISNET M, THOMAS M, NICOLAOS A. Adsorption and reaction over HFAU zeolites of thiophene in liquid hydrocarbon solutions[J]. Appl Catal A:Gen, 2004, 263(1):49-61. doi: 10.1016/j.apcata.2003.11.039 [12] SHI Y C, ZHANG W, ZHANG H X, TIAN F P, JIA C Y, CHEN Y Y. Effect of cyclohexene on thiophene adsorption over NaY and LaNaY zeolites[J]. Fuel Process Technol, 2013, 110:24-32. doi: 10.1016/j.fuproc.2013.01.008 [13] SELVARAJ M, LEE T G. Room temperature synthesis of diphenylmethane over novel mesoporous Lewis acid catalysts[J]. J Mol Catal:A Chem, 2006, 243(2):176-182 doi: 10.1016/j.molcata.2005.08.020 [14] WANG Z C, JIANG Y J, LAFON O, TREBOSC J, KIM K D, STAMPF C, BAIKER A, AMOUREUS J-P, HUANG J. Brønsted acid sites based on penta-coordinated aluminum species[J]. Nat Commun, 2016, 7:13820. doi: 10.1038/ncomms13820 [15] HU W, LUO Q, SU Y C, CHEN L, YUE Y, YE C H, DENG F. Acid sites in mesoporous Al-SBA-15 material as revealed by solid-state NMR spectroscopy[J]. Microporous Mesoporous Mater, 2006, 92(1):22-30. [16] GURINOV A A, ROZHKOVA Y A, ZUKALA, CEJKA J, SHENDEROVICH I G. Mutable lewis and Brønsted acidity of aluminated SBA-15 as revealed by NMR of adsorbed pyridine-15N[J]. Langmuir, 2011, 27(19):12115. doi: 10.1021/la2017566 [17] TANG K, SONG L J, DUAN L H, LI X Q, GUI J Z, SUN Z L. Deep desulfurization by selective adsorption on a heteroatoms zeolite prepared by secondary synthesis[J]. Fuel Process Technol, 2008, 89(1):1-6. doi: 10.1016-j.fuproc.2007.06.002/ [18] 秦玉才, 高雄厚, 裴婷婷, 郑兰歌, 王琳, 莫周胜, 宋丽娟.噻吩在稀土离子改性Y型分子筛上吸附与催化转化研究[J].燃料化学学报, 2013, 41(7):889-896. doi: 10.3969/j.issn.0253-2409.2013.07.017QIN Yu-cai, GAO Xiong-hou, PEI Ting-ting, ZHENG Lan-ge, WANG Lin, MO Zhou-sheng, SONG Li-juan. Adsorption and catalytic conversion of thiophene on Y-type zeolites modified with rare-earth metal ions[J]. J Fuel Chem Technol, 2013, 41(7):889-896. doi: 10.3969/j.issn.0253-2409.2013.07.017 [19] 张畅, 秦玉才, 高雄厚, 张海涛, 莫周胜, 初春雨, 张晓彤, 宋丽娟. Ce改性对Y型分子筛酸性及其催化转化性能的调变机制[J].物理化学学报, 2015, 31(2):344-352. http://d.old.wanfangdata.com.cn/Periodical/wlhxxb201502021ZHANG Chang, QIN Yu-cai, GAO Xiong-hou, ZHANG Hai-tao, MO Zhou-sheng, CHU Chun-yu, ZHANG Xiao-tong, SONG Li-juan. Modulation mechanisms of the acidity and catalytic properties of Y zeolites modified by cerium cations[J]. Acta Phys-Chim Sin, 2015, 31(2):344-352. http://d.old.wanfangdata.com.cn/Periodical/wlhxxb201502021 [20] 祖运, 秦玉才, 高雄厚, 莫周胜, 张磊, 张晓彤, 宋丽娟.催化裂化条件下噻吩与改性Y分子筛的作用机制[J].燃料化学学报, 2015, 43(7):862-869. doi: 10.3969/j.issn.0253-2409.2015.07.012ZU Yun, QIN Yu-cai, GAO Xiong-hou, MO Zhou-sheng, ZHANG Lei, ZHANG Xiao-tong, SONG Li-juan. Mechanisms of thiophene conversion over the modified Y zeolites under catalytic cracking conditions[J]. J Fuel Chem Technol, 2015, 43(7):862-869. doi: 10.3969/j.issn.0253-2409.2015.07.012 [21] 丁润东, 祖运, 周传行, 王焕, 莫周胜, 秦玉才, 孙兆林, 宋丽娟. CuNaY分子筛的有效吸附位与其脱硫性能的关联性研究[J].燃料化学学报, 2018, 46(4):451-458. doi: 10.3969/j.issn.0253-2409.2018.04.010DING Run-dong, ZU Yun, ZHOU Chuan-hang, WANG Huan, MO Zhou-sheng, QIN Yu-cai, SUN Zhao-lin, SONG Li-juan. Insight into the correlation between effective adsorption sites and adsorption desulfurization performance of CuNaY zeolite[J]. J Fuel Chem Technol, 2018, 46(4):451-458. doi: 10.3969/j.issn.0253-2409.2018.04.010 [22] DUBÉ D, ROYR S, ON D T, BELAND F, KALIAGUINE S. Aluminum chloride grafted mesoporous molecular sieves as alkylation catalysts[J]. Microporous Mesoporous Mater, 2005, 79:137-144. doi: 10.1016/j.micromeso.2004.11.002 [23] GALLO J M R, BISIO C, GATTI G, MARCHESE L, PASTORE H O. Physicochemical characterization and surface acid properties of mesoporous[Al]-SBA-15 obtained by direct synthesis[J]. Langmuir, 2010, 26(8):5791-5800. doi: 10.1021/la903661q [24] MARQUES J P, GENER I, AYRAULT P, BORDADO J C, LOPES J M, RIBEIRO F R, GUISNET M. Infrared spectroscopic study of the acid properties of dealuminated BEA zeolites[J]. Microporous Mesoporous Mater, 2003, 60:251-262. doi: 10.1016/S1387-1811(03)00382-2 [25] EMIES C A. Determination of integrated molar extinction coefficients for infrared absorption bands of pyridine adsorbed on solid acid catalysts[J]. J Catal, 1993, 141:347-354. doi: 10.1006/jcat.1993.1145 -





 下载:
下载: