Removal of elemental mercury in flue gas with PMS solution catalyzed by Co doped BiFeO3
-
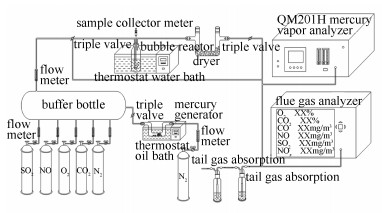
摘要: 采用酒石酸溶胶凝胶法制备了一系列的钴掺杂的铁酸铋催化剂(BiFe1-xCoxO3,x=5%-20%,x为Co/CoFe物质的量比),借助于X射线衍射(XRD)、扫描电子显微镜(SEM)、比表面积(BET)、磁强振动计(VSM)、X射线光电子能谱(XPS)等手段对催化剂进行表征。在自制鼓泡反应器内,利用钴掺杂铁酸铋活化过一硫酸氢钾(PMS),开展了模拟烟气中单质汞脱除实验,获得反应的最佳条件。当钴掺杂量为10%,催化剂用量为0.5 g/L,PMS浓度为3.9 mmol/L,溶液初始pH值为8,反应温度70 ℃时,反应100 min内Hg0的平均脱除效率达89.36%。以乙醇和叔丁醇为淬灭剂,证明了·OH和SO4·-为Hg0催化氧化的活性物种,且SO4·-起主要作用,并结合XPS分析结果推测了脱汞反应机理。Abstract: A aseries of Co-doped BiFeO3 magnetic catalysts(BiFe1-xCoxO3, x=5%-20%) were synthesized by the tartaric acid sol-gel method, and the prepared catalysts were characterized using X-ray powder diffraction (XRD), Brunauer Emmett Teller (BET) technique, vibration sample magnetometer (VSM) and X ray photoelectron spectroscopy(XPS). The catalytic activity of Co doped BiFeO3 to activate Peroxymonosulfate (PMS) was evaluated at a self-designed bubbling reactor. The effects of Co ration in catalyst, dosage of the catalyst, PMS concentration, and solution pH and reaction temperature on the removal of elemental mercury were investigated systematically, and the optimum conditions were obtained. The result indicates that the average removal efficiency of elemental mercury reaches 89.36% within 100 min under the following condition:70℃, 10% doping Co, 3.9 mmol/L PMS concentration, 0.5 g/L catalyst dosage and pH 8. Moreover, it is testified that SO4·- and·OH are the active species when Hg0 is oxidized to Hg2+, where the tert-butyl alcohol and ethyl alcohol are used as quenchers. Finally, the mechanisms of mercury removal with PMS solution catalyzed by BiFe0.9Co0.1O3 are speculated on the basis of XPS results.
-
Key words:
- peroxymonosulfate /
- Co doped BiFeO3 /
- sulfate radical /
- mercury
-
表 1 平行实验结果
Table 1 Results of parallel test
1 2 3 4 5 Average value Variance Removal efficiency /% 91.32 89.94 87.96 89.78 87.98 89.36 1.43 表 2 反应前后催化剂XPS分峰拟合分析
Table 2 Results of XPS peak-differentiation-imitating analysis before and after reaction
Element Before reaction After reaction binding energy E/eV valence state percentage/% binding energy E/eV valence state percentage/% Co 779.1 Co2+ 41.99 779.4 Co3+ 37.76 780.1 Co2+ 38.99 780.3 Co3+ 39.10 781.3 Co2+ 19.02 781.3 Co2+ 23.14 Fe 709.8 Fe2+ 60.26 709.8 Fe2+ 71.14 711.3 Fe3+ 15.28 711.3 Fe3+ 7.95 712.9 Fe3+ 24.46 712.9 Fe3+ 20.91 O 529.8 lattice oxygen 51.39 529.8 lattice oxygen 45.77 531.2 hydroxyl oxygen 48.61 531.2 hydroxyl oxygen 52.45 oxygen oxygen -
[1] ZHAO Y, HAO R L, GUO Q. A novel pre-oxidation method for elemental mercury removal utilizing a complex vaporized absorbent[J].J Hazard Mater, 2014, 280:118-126. doi: 10.1016/j.jhazmat.2014.07.061 [2] LIU Y X, WANG Y, WANG Q, PAN J F, ZHANG Y, ZHOU J, ZHANG J. A study on removal of elemental mercury in flue gas using Fenton solution[J]. J Hazard Mater, 2015, 292:164-172. doi: 10.1016/j.jhazmat.2015.03.027 [3] LIU Y X, ZHOU J F, ZHANG Y C, PAN J F, WANG Q, ZHANG J. Removal of Hg0 and simultaneous removal of Hg0/SO2/NO in flue gas using two Fenton-like reagents in a spray reactor[J]. Fuel, 2015, 145:180-188. doi: 10.1016/j.fuel.2014.12.084 [4] ZHOU C, SUN L, ZHANG A, WU X, MA C, SU S. Fe3-x CuxO4 as highly active heterogeneous Fenton-like catalysts toward elemental mercury removal[J]. Chemosphere, 2015, 125:16-24. doi: 10.1016/j.chemosphere.2014.12.082 [5] ZHOU C, WANG B, MA C, SONG Z J, ZENG Z. Gaseous elemental mercury removal through heterogeneous Fenton-like processes using novel magnetically separable Cu0.3Fe2.7-xTixO4catalysts[J]. Fuel, 2015, 161(2):254-261. http://chianti.ucsd.edu/cyto_web/plugins/pluginjardownload.php?id=590 [6] JI Y, KONG D, LU J, HAO J, KANG F, YIN X. Cobalt catalyzed peroxymonosulfate oxidation of tetrabromobisphenol A:Kinetics, reaction pathways, and formation of brominated by-products[J]. J Hazard Mater, 2016, 313:229-237. doi: 10.1016/j.jhazmat.2016.04.033 [7] WANG Y, ZHOU L, DUAN X, SUN H, TIN EL, JIN W. Photochemical degradation of phenol solutions on Co3O4nanorods with sulfate radicals[J]. Catal Today, 2015, 258:576-584. doi: 10.1016/j.cattod.2014.12.020 [8] DENG J, SHAO Y, GAO N, TAN C, ZHAO S, HU X. CoFe2O4 magnetic nanoparticles as a highly active heterogeneous catalyst of oxone for the degradation of diclofenac in water[J]. J Hazard Mater, 2013, 262:836-844. doi: 10.1016/j.jhazmat.2013.09.049 [9] FENG Y, LIU J, WU D, ZHOU Z, DENG Y, ZHANG T. Efficient degradation of sulfamethazine with CuCo2O4 spinel nanocatalysts for peroxymonosulfate activation[J].Chem Eng J, 2015, 280:514-524. doi: 10.1016/j.cej.2015.05.121 [10] JAAFARZADEG N, GHANBARI F, AHMADI M. Catalytic degradation of 2, 4-dichlorophenoxyacetic acid (2, 4-D) by nano-Fe2O3 activated peroxymonosulfate:Influential factors and mechanism determination[J]. Chemosphere, 2016, 169:568-576. doi: 10.1021/es025896h?source=chemport [11] AN J, ZHU L, WANG N, SONG Z, YANG Z, DU D, TANG H. Photo-Fenton-like degradation of tetrabromobisphenol A with graphene BiFeO3 composite as a catalyst[J]. Chem Eng J, 2013, 219:225-237. doi: 10.1016/j.cej.2013.01.013 [12] GU Y H, ZHAO J G, ZHANG W Y, LIU S, GE S P, CHEN W P, ZHANG Y. Improved ferromagnetism and ferroelectricity of La and Co co-doped BiFeO3 ceramics with Fe vacancies[J].Ceram Int, 2016, 42(7):8863-8868. doi: 10.1016/j.ceramint.2016.02.134 [13] ZHANG L, YANG X, HAN E, ZHAO L, LIAN J. Reduced graphene oxide wrapped Fe3O4-Co3O4 yolk-shell nanostructures for advanced catalytic oxidation based on sulfate radicals[J]. Appl Surf Sci, 2017, 396:945-954. doi: 10.1016/j.apsusc.2016.11.066 [14] KHARISOV B I, DIAS H V R, KHARISSOVA O V. Mini-review:Ferrite nanoparticles in the catalysis[J]. Arab J Chem, 2014, 1-13. https://www.sciencedirect.com/science/article/pii/S1878535214002901 [15] REN Y, LIN L, MA J, YANG J, FENG J, FAN Z. Sulfate radicals induced from peroxymonosulfate by magnetic ferrospinel MFe2O4, (M=Co, Cu, Mn, and Zn) as heterogeneous catalysts in the water[J]. Appl Catal B:Environ, 2015, 165:572-578. doi: 10.1016/j.apcatb.2014.10.051 [16] CAI C, ZHANG H, ZHONG X, HOU L. Ultrasound enhanced heterogeneous activation of peroxymonosulfate by a bimetallic Fe-Co/SBA-15 catalyst for the degradation of orange Ⅱ in water[J]. J Hazard Mater, 2014, 283:70-75. https://www.sciencedirect.com/science/article/pii/S030438941400716X [17] MADHAVAN J, MARUTHAMUTHU P, MURUGESAN S, ANANDAN S. Kinetic studies on visible light-assisted degradation of acid red 88 in presence of metal-ion coupled oxone reagent[J].Appl Catal B:Environ, 2008, 83(1/2):8-14. https://www.sciencedirect.com/science/article/pii/S0926337308000568 [18] XU L, WANG J. Magnetic nanoscaled Fe3O4/CeO2 composite as an efficient Fenton-like heterogeneous catalyst for degradation of 4-chlorophenol[J]. Environ Sci Technol, 2016, 46(18):10145-10153. https://www.sciencedirect.com/science/article/pii/S0925838817324271 [19] YU Z, WANG W, SONG L, LU L, WANG Z, JIANQ X, DONG C, QIU R. Acceleration comparison between Fe2+/H2O2, and Co2+/oxone for decolouration of azo dyes in homogeneous systems[J]. Chem Eng J, 2013, 234:475-483. doi: 10.1016/j.cej.2013.08.013 [20] XU X, YE Q, TANG T, WANG D. Hg0 oxidative absorption by K2S2O8solution catalyzed by Ag+ and Cu2+[J]. J Hazard Mater, 2008, 158(2/3):410-416. http://qiserver.ugr.es/cod/result.php?CODSESSION=rjgq0ukolm3upn5487v24ls4i1l9ten9&count=1000&page=0&order_by=sg&order=asc [21] NIKSA S, HELBLE J J, FUJIWARA N. Kinetic modeling of homogeneous mercury oxidation:The importance of NO and H2O in predicting oxidation in coal-derived systems[J]. Environ Sci Technol, 2001, 35(18):3701-3706. doi: 10.1021/es010728v [22] GUAN Y H, MA J, REN Y M, LIU Y L, LIN LQ, ZHANG C. Efficient degradation of atrazine by magnetic porous copper ferrite catalyzed peroxymonosulfate oxidation via the formation of hydroxyl and sulfate radicals[J]. Water Res, 2013, 47(14):5431-5438. doi: 10.1016/j.watres.2013.06.023 [23] ZOU J, MA J, CHEN L, LI X, GUAN Y, XIE P, PAN C. Rapid acceleration of ferrousiron/peroxymonosulfate oxidation of organic pollutants by promoting Fe(Ⅲ)/Fe(Ⅱ) cycle with hydroxylamine[J], Environ Sci Technol, 2013, 47(20):11685-11691. doi: 10.1021/es4019145 [24] ZHANG T, ZHU H, CROUE J P. Production of sulfate radical from peroxymonosulfate induced by a magnetically separable CuFe2O4 spinel in water:efficiency, stability, and mechanism[J]. Environ Sci Technol, 2013, 47(6):2784-2791. doi: 10.1021/es304721g [25] WU Q, ZHANG H, ZHOU L, BAO C, ZHU H, ZHANG Y. Synthesis and application of rGO/CoFe2O4 composite for catalytic degradation of methylene blue on heterogeneous Fenton-like oxidation[J]. J Taiwan Inst Chem Eng, 2016, 67:484-494. doi: 10.1016/j.jtice.2016.08.004 [26] XU Y, AI J, ZHANG H. The mechanism of degradation of bisphenol A using the magnetically separable CuFe2O4/peroxymonosulfate heterogeneous oxidation process[J]. J Hazard Mater, 2016, 309:87-96. doi: 10.1016/j.jhazmat.2016.01.023 [27] HU P, LONG M. Cobalt-catalyzed sulfate radical-based advanced oxidation:A review on heterogeneous catalysts and applications[J]. Appl Catal B:Environ, 2016, 181:103-117. doi: 10.1016/j.apcatb.2015.07.024 [28] TAN C, GAO N, DENG Y, DENG J, ZHOU S, LI J, XIN X. Radical induced degradation of acetaminophen with Fe3O4 magnetic nanoparticles as heterogeneous activator of peroxymonosulfate[J]. J Hazard Mater, 2014, 276:452-460. doi: 10.1016/j.jhazmat.2014.05.068 [29] ERIC G H, SUDIPTA S, WILLIAM T S. Fenton-like reaction catalyzed by the rare earth Inner transition metal cerium[J]. Environ Sci Technol, 2008, 42(13):5014-5019. doi: 10.1021/es8001508 [30] ZHAO Y, HAO R, ZHANG P, ZHOU S. An integrative process for Hg0 removal using vaporized H2O2/Na2S2O8[J]. Fuel, 2014, 136:113-121. doi: 10.1016/j.fuel.2014.07.046 [31] SOMMAR J, KATARINA D, STROMBERG D, FENG X. A kinetic study of the gas-phase reaction between the hydroxyl radical and atomic mercury[J]. Atmos Environ, 2001, 35:3049-3054. doi: 10.1016/S1352-2310(01)00108-X -





 下载:
下载: