Performance of the platinum nanoparticles supported on three-dimensional graphene as electro-catalyst for methanol electro-oxidation
-
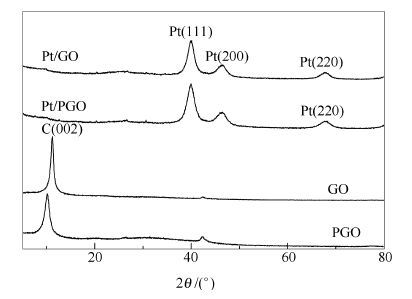
摘要: 喷雾干燥法制备具有三维结构的氧化石墨烯(PGO),在其表面进一步负载活性成分Pt,得到纳米Pt/PGO复合催化剂。采用X射线粉末衍射(XRD)、透视电镜(TEM)和扫描电镜(SEM)等对催化剂的形貌和结构进行表征。结果表明,PGO具有类似于长4-6 μm和宽2.0-3.0 μm的三维纸团结构,平均粒径为4.2 nm的Pt纳米粒子均匀分布在其表面。采用循环伏安和计时电流法研究了在酸性溶液中催化剂对甲醇的电催化氧化性能。结果表明,Pt/PGO催化剂对甲醇呈现出更高的电催化氧化活性和稳定性。PGO所具有的三维结构和双功能作用机理有利于甲醇在铂表面的电催化氧化过程的发生。Abstract: Three-dimensional graphene (PGO) was synthesized by spay-drying method.Pt/PGO catalyst was prepared through the improved liquid phase reduction method.The samples were characterized by XRD, BET and TEM.The results indicated that PGO was a three-dimensional crumpled structure material with 4-6 μm in length and 2.0-3.0 μm in width and the Pt nanoparticles with 4.2 nm were highly dispersed on the surface of PGO.The electro-catalytic activity and stability of the catalysts towards methanol electro-oxidation were investigated using cyclic voltammetry and chronoamperometry.The as-prepared Pt/PGO catalyst exhibits higher electro-catalytic activity and good stability during methanol electro-oxidation in comparison to Pt/GO catalysts.The enhanced catalytic performance is attributed to the high specific surface area of the 3D formation and utilization efficiency of Pt during methanol electro-oxidation.
-
Key words:
- direct methanol fuel cell /
- methanol electro-oxidation /
- graphene /
- electro-catalysis /
- spay-drying
-
表 1 Pt/PGO和Pt/GO催化剂对甲醇氧化性能
Table 1 Comparison of the activity of alcohols oxidation on Pt / PGO and Pt / GO catalysts
Catalyst Onset potential E/V ECSA A/(m2·g-1) If/(mA·mg-1Pt) If/Ib Pt/PGO 0.40 89.5 756 1.00 Pt/GO 0.45 49.6 350 0.95 -
[1] ZHOU Y, HU XC, LIU XH, WEN H R. Core-shell hierarchical WO2/WO3 microspheres as an electrocatalyst support for methanol electrooxidation[J]. Chem Commun, 2015, 51(83):15297-15299. doi: 10.1039/C5CC06603D [2] 周阳, 褚有群, 刘委明, 马淳安. 纳米三氧化钨修饰碳纳米管载铂催化剂对甲醇氧化的电催化性能[J]. 物理化学学报, 2013, 29(2):287-292. http://www.cnki.com.cn/Article/CJFDTOTAL-WLHX201302011.htm(ZHOU Yang, CHU You-qun, LIU Wei-ming, MA Chun-an. Nano-WO3 modified carbon nanotube supported Pt catalysts and their electro-catalytic activity for methanol electro-oxidation[J]. Acta Phys-Chim Sin, 2013, 29(2):287-292.) http://www.cnki.com.cn/Article/CJFDTOTAL-WLHX201302011.htm [3] LUO J Y, JANG H D, SUN T. Compression and aggregation-resistant particles of crumpled soft sheets[J]. ACS Nano, 2011, 5(11):8943-8949. doi: 10.1021/nn203115u [4] 俞贵艳, 陈卫祥, 赵杰. PtNi/C催化剂的合成及其对甲醇氧化的电催化性能[J]. 浙江大学学报:工学版, 2007, 41(12):2107-2111. http://www.cnki.com.cn/Article/CJFDTOTAL-ZDZC200712033.htm(YU Gui-yan, CHEN Wei-xiang, ZHAO Jie. Synthesis of PtNi/C electrocatalysts and their electrocatalytic performance for methanol electrooxidation[J]. J Zhejiang Univ:Eng Sci, 2007, 41(12):2107-2111.) http://www.cnki.com.cn/Article/CJFDTOTAL-ZDZC200712033.htm [5] JANG H D, KIM S K, CHANG H. Three-dimensional crumpled graphene-based platinum-gold alloy nanoparticle composites as superior electrocatalysts for direct methanol fuel cells[J]. Carbon, 2015, 93:869-877. doi: 10.1016/j.carbon.2015.06.009 [6] LI Y C, ZHANG L, HUB ZF. Synthesis of 3D structured graphene as a high performance catalyst support for methanol electro-oxidation[J]. Nanoscale, 2015, 7(25):10896-10902. doi: 10.1039/C5NR02766G [7] 汪建德, 彭同江, 鲜海洋, 孙红娟. 三维还原氧化石墨烯/聚苯胺复合材料的制备及其超级电容性能[J]. 物理化学学报, 2015, 31(1):90-98. http://www.cnki.com.cn/Article/CJFDTOTAL-WLHX201501015.htm(WANG Jian-de, PENG Tong-jiang, XIAN Hai-yang, SUN Hong-juan. Preparation and supercapacitive performance of three-dimensional reduced graphene oxide/polyaniline composite[J].Acta Phys-Chim Sin, 2015, 31(1):90-98.) http://www.cnki.com.cn/Article/CJFDTOTAL-WLHX201501015.htm [8] NIU Z Q, CHEN J, HNG H H. A leavening strategy to prepare reduced graphene oxide foams[J]. Adv Mater, 2012, 24(30):4144-4150. doi: 10.1002/adma.201200197 [9] YAVARI F, CHEN Z P, THOMAS A V. High sensitivity gas detection using a macroscopic three-dimensional graphene foam network[J]. Sci Rep, 2011, 166. https://www.researchgate.net/profile/Fazel_Yavari/publication/221852464_High_Sensitivity_Gas_Detection_Using_a_Macroscopic_Three-Dimensional_Graphene_Foam_Network/links/09e4150884a7ac57da000000.pdf?inViewer=true&pdfJsDownload=true&disableCoverPage=true&origin=publication_detail [10] WS H, RE O. Preparation of graphitic oxide[J]. J Am Chem Soc, 1958, 80(6):1339. doi: 10.1021/ja01539a017 [11] 温祝亮, 杨苏东, 宋启军. 石墨烯负载高活性Pd催化剂对乙醇的电催化氧化[J]. 物理化学学报, 2010, 26(6):1570-1574. http://www.cnki.com.cn/Article/CJFDTOTAL-WLHX201006019.htm(WEN Zhu-liang, YANG Su-dong, SONG Qi-jun. High activity of Pd/Graphene catalysts for ethanol electro-catalytic oxidation[J]. Acta Phys-Chim Sin, 2010, 26(6):1570-1574.) http://www.cnki.com.cn/Article/CJFDTOTAL-WLHX201006019.htm [12] ZHOU Y, HU X C, XIAO Y J, SHU Q. Platinum nanoparticles supported on hollow mesoporous tungsten trioxide microsphere as electrocatalyst for methanol oxidation[J]. Electrochim Acta, 2013, 111:588-592. doi: 10.1016/j.electacta.2013.08.057 [13] HU X C, ZHOU Y, WEN H R, ZHONG H M. Hierarchical hollow tungsten trioxide sphere as an electrocatalyst support for formic acid electrooxidation[J]. J Electrochem. Soc, 2014, 161(5):F583-F587. doi: 10.1149/2.008405jes [14] PRABHURAM J, ZHAO T S, TANG Z K. Multiwalled carbon nanotube supported PtRu for the anode of direct methanol fuel cells[J]. J Phys Chem B, 2006, 110(11):5245-5252. doi: 10.1021/jp0567063 [15] POZIO A, GIORGI L, DE FRANCESCO M. Membrane electrode gasket assembly (MEGA) technology for polymer electrolyte fuel cells[J]. J Power Sources, 2002, 112(2):491-496. doi: 10.1016/S0378-7753(02)00458-5 [16] 胡仙超, 胡建冠, 孙洁, 李国华. 介孔碳化钨负载铂催化剂对甲醇氧化的电催化性能[J]. 无机材料学报, 2013, 28(12):1286-1290. http://www.cnki.com.cn/Article/CJFDTOTAL-WGCL201312003.htm(HU Xian-chao, HU Jian-guang, SUN Jie, LI Guo-hua. Mesoporous tungsten carbide supported Pt and their electro catalytic activity for methanol electro-oxidation[J]. J Inorg Mater, 2013, 28(12):1286-1290.) http://www.cnki.com.cn/Article/CJFDTOTAL-WGCL201312003.htm [17] HU X C, ZHOU Y, ZHONG H M, WEN H R. Synthesis of hierarchical hollow tungsten trioxide sphere and its evaluation as an electrocatalyst support for methanol oxidation[J]. J Solid State Electrochem, 2015, 19(2):315-320. doi: 10.1007/s10008-014-2416-0 [18] JI Y C, YU L, HOU M, SHEN L M, WANG Z Q. A three dimensional graphene/Pt catalyst fabricated by particle-constructing method and the catalytic activity for methanol oxidation[J]. Int J Hydrogen Energy, 2016, 41(44):20078-20084. doi: 10.1016/j.ijhydene.2016.09.119 [19] ZHANG J, MA L, GAN M Y. Well-dispersed platinum nanoparticles supported on hierarchical nitrogen-doped porous hollow carbon spheres with enhanced activity and stability for methanol electrooxidation[J]. J Power Sources, 2015, 288:42-52. doi: 10.1016/j.jpowsour.2015.04.109 [20] SHARMA S, GANGULY A, PAPAKONSTANTINOU P. Rapid microwave synthesis of CO tolerant reduced graphene oxide-supported platinum electrocatalysts for oxidation of methanol[J]. J Phys Chem C, 2010, 114(45):19459-19466. doi: 10.1021/jp107872z [21] KUA J, GODDARD W A. Oxidation of methanol on 2nd and 3rd row Group VⅢ transition metals (Pt,Ir,Os,Pd,Rh,and Ru):Application to direct methanol fuel cells[J]. J Am Chem Soc, 1999, 121(47):10928-10941. doi: 10.1021/ja9844074 -





 下载:
下载: