Influence of preparation method on the structure of NiCo/MgO catalyst and its performance in the reforming of CH4 with CO2
-
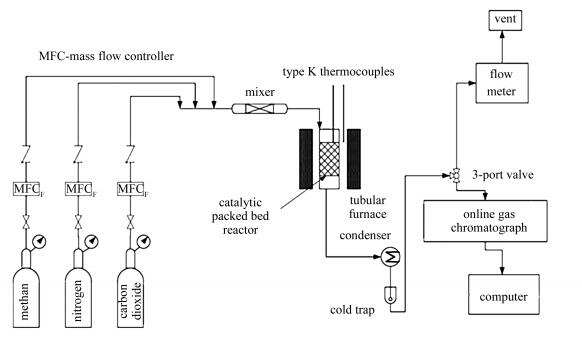
摘要: 为进一步提高镍基催化剂的抗积炭能力,增强其甲烷二氧化碳重整反应性能,采用沉积沉淀法(DP)、共沉淀法(CP)和共浸渍法(CI)制备了NiCo/MgO催化剂。结合现代仪器分析表征技术,研究了制备方法对NiCo/MgO催化剂结构和抗积炭能力的影响。结果表明,与共沉淀法相比,沉积沉淀法制备过程为Ni2+和Co2+的完全水解沉淀提供了碱性环境,粒子的成核和生长速率相对较快,不存局部过饱和现象,所制备的催化剂具有良好的还原性、较小的颗粒粒径(9.7 nm)、良好的Ni/Co分散度(10.4%)和大的比表面积(68.1 m2/g),从而具有优良的抗积炭性能。对于甲烷二氧化碳重整,DP催化剂上CH4和CO2转化率保持在88%和92%,与800℃下的热力学平衡转化率相近;同时,H2收率比CP和CI催化剂分别高约10%和43%,CO收率比CP和CI催化剂分别高约13%和42%,且稳定性更好。Abstract: To enhance the performance of nickel-based catalysts in the reforming of CH4 with CO2 and alleviate the coke deposition, a series of NiCo/MgO catalysts were prepared by different methods, viz., deposition-precipitation (DP), co-precipitation method (CP) and co-impregnation (CI); the influence of preparation method on the structure and performance of NiCo/MgO catalyst was then investigated. The results show that during the deposition-precipitation process, CO(NH2)2 as the precipitant could created an alkaline atmosphere for the complete hydrolysis of Ni2+ and Co2+ ions, leading to a relatively fast nucleation and growth of active species; however, oversaturation may occur during the co-precipitation process with NaOH and Na2CO3 as the precipitants. In comparison with the catalysts prepared by CP and CI, the NiCo/MgO-DP catalyst is provided with superior reduction capacity, smaller particle size (9.7 nm), higher Ni/Co dispersion (10.4%) and larger specific surface area (68.1 m2/g) and then exhibits better resistance to coke deposition. Over the DP catalyst, the conversions of CH4 and CO2 at 800 ℃ reach 88% and 92%, respectively, much higher than those over the CP and CI catalysts; moreover, the DP catalyst also gives much higher yield of H2 and CO as well as better stability for methane reforming with CO2.
-
Key words:
- methane reforming with CO2 /
- preparation method /
- Ni /
- Co /
- MgO /
- deposition-precipitation /
- co-precipitation method /
- co-impregnation
-
表 1 不同制备方法NiCo/MgO催化剂的元素组成
Table 1 Element composition of the NiCo/MgO catalysts synthesized by different methods

表 2 不同制备方法催化剂金属分散度与颗粒粒径
Table 2 Metal dispersion and particle size of the NiCo/MgO catalysts synthesized by different methods

表 3 不同制备方法催化剂的比表面积和孔结构
Table 3 BET specific surface area and pore structure of the NiCo/MgO catalysts synthesized by different methods

-
[1] FENG X, FENG J, LI W. CO2 reforming of CH4 over a highly active and stable ni-mg-al catalyst[J]. Int J Hydrogen Energy, 2017, 42(5):3036-3042. doi: 10.1016/j.ijhydene.2016.09.205 [2] FENG J, DING Y, GUO Y, LI X, LI W. Calcination temperature effect on the adsorption and hydrogenated dissociation of CO2 over the NiO/MgO catalyst[J]. Fuel, 2013, 109(7):110-115. http://www.sciencedirect.com/science/article/pii/S0016236112006643 [3] 张盼艺, 郭芳, 许俊强, 陈志, 李军.基于强抗积碳的CO2重整镍基催化剂的研究进展[J].硅酸盐学报, 2016, 44(4):620-626. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=gxyb201604026&dbname=CJFD&dbcode=CJFQZHANG Pan-yi, GUO Fang, XU Jun-qiang, CHEN Zhi, LI Jun. Progress of coke resistant ability research of Ni-based catalysts for CO2 reforming of methane[J]. J Chin Ceram Soc, 2016, 44(4):620-626. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=gxyb201604026&dbname=CJFD&dbcode=CJFQ [4] PAKHARE D, SPIVEY J. A review of dry (CO2) reforming of methane over noble metal catalysts[J]. Chem Soc Rev, 2014, 43(22):7813. doi: 10.1039/C3CS60395D [5] 王明智, 张秋林, 张腾飞, 王一茹. Ni基甲烷二氧化碳重整催化剂研究进展[J].化工进展, 2015, 34(8):3027-3034. http://www.cnki.com.cn/Article/CJFDTOTAL-HGJZ201508023.htmWANG Zhi-ming, ZHANG Qiu-lin, ZHANG Teng-fei, WANG Yi-ru. Advance in Ni-based catalysts for the carbon dioxide reforming of methane[J]. Chem Ind Eng Prog, 2015, 34(8):3027-3034. http://www.cnki.com.cn/Article/CJFDTOTAL-HGJZ201508023.htm [6] 付晓娟, 曾尚红, 苏海全.用于甲烷二氧化碳重整新型催化材料的研究进展[J].化工进展, 2012, 31(S1):168-175. http://www.cnki.com.cn/Article/CJFDTOTAL-HGJZ2012S1036.htmFU Xiao-juan, ZENG Shang-hong, SU Hai-quan. Advance in catalytic materials for CO2 reforming of methane:A review[J]. Chem Ind Eng Prog, 2012, 31(S1):168-175. http://www.cnki.com.cn/Article/CJFDTOTAL-HGJZ2012S1036.htm [7] RUCKENSTEIN E, HANG Hu Y. Role of support in CO2 reforming of CH4 to syngas over ni catalysts[J]. J Catal, 1996, 162(2):230-238. doi: 10.1006/jcat.1996.0280 [8] 霍苗苗, 李琳, 赵欣, 张煜华, 李金林.氮化SBA-16负载镍基催化剂的制备及其对甲烷二氧化碳重整反应的催化性能[J].燃料化学学报, 2017, 45(2):172-181. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18976.shtmlHUO Miao-miao, LI Lin, ZHAO Xin, ZHANG Yu-hua, LI Jin-lin. Synthesis of ni-based catalysts supported on nitrogen-incorporated SBA-16 and their catalytic performance in the reforming of methane with carbon dioxide[J]. J Fuel Chem Technol, 2017, 45(2):172-181. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18976.shtml [9] 王冰, 郭聪秀, 王英勇, 靳国强, 郭向云. Ni-Smx/SiC催化剂甲烷二氧化碳重整性能研究[J].燃料化学学报, 2016, 44(5):587-596. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18832.shtmlWANG Bin, GUO Cong-xiu, WANG Ying-yong, JIN Guo-qiang, GUO Xiang-yun. Performance of Ni-Smx/SiC catalysts for CO2 reforming of CH4[J]. J Fuel Chem Technol, 2016, 44(5):587-596. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18832.shtml [10] WU H, LA Parola V, PANTALEO G, PULEO F, VENEZIA A, LIOTTA L. Ni-based catalysts for low temperature methane steam reforming:Recent results on Ni-Au and comparison with other bi-metallic systems[J]. Catalysts, 2013, 3(2):563-583. doi: 10.3390/catal3020563 [11] GARCIA-DIEGUEZ M, PIETA I S, HERRERA M C, LARRUBIA M A, ALEMANY L J. Improved Pt-Ni nanocatalysts for dry reforming of methane[J]. Appl Catal A:Gen, 2010, 377(1/2):191-199. http://www.sciencedirect.com/science/article/pii/S0926860X1000075X [12] FAN M-S, ABDULLAH A Z, BHATIA S. Catalytic technology for carbon dioxide reforming of methane to synthesis gas[J]. ChemCatChem, 2009, 1(2):192-208. doi: 10.1002/cctc.v1:2 [13] SHESHKOT F, SEROV Y M. Bimetallic systems containing Fe, Co, Ni, and Mn nanoparticles as catalysts for the hydrogenation of carbon oxides[J]. Russ J Phys Chem A, 2012, 86(2):283-288. doi: 10.1134/S0036024412020264 [14] ZHANG F, WANG N, YANG L, LIM, HUANG L. Ni-Co bimetallic mgo-based catalysts for hydrogen production via steam reforming of acetic acid from bio-oil[J]. Int J Hydrogen Energy, 2014, 39(32):18688-18694. doi: 10.1016/j.ijhydene.2014.01.025 [15] MIRZAEI F, REZAEI M, MESHKANI F. Coprecipitated Ni-Co bimetallic nanocatalysts for methane dry reforming[J]. Chem Eng Technol, 2014, 37(6):973-978. doi: 10.1002/ceat.v37.6 [16] ZHANG J, WANG H, DALAI A K. Development of stable bimetallic catalysts for carbon dioxide reforming of methane[J]. J Catal, 2007, 249(2):300-310. doi: 10.1016/j.jcat.2007.05.004 [17] ZHANG J, WANG H, DALAI A K. Effects of metal content on activity and stability of Ni-Co bimetallic catalysts for CO2 reforming of CH4[J]. Appl Catal A:Gen, 2008, 339(2):121-129. doi: 10.1016/j.apcata.2008.01.027 [18] XU J, ZHOU W, LI Z, WANG J, MA J. Biogas reforming for hydrogen production over nickel and cobalt bimetallic catalysts[J]. Int J Hydrogen Energy, 2009, 34(16):6646-6654. doi: 10.1016/j.ijhydene.2009.06.038 [19] WANG Z, WANG C, CHEN S, LIU Y. Co-Ni bimetal catalyst supported on perovskite-type oxide for steam reforming of ethanol to produce hydrogen[J]. Int J Hydrogen Energy, 2014, 39(11):5644-5652. doi: 10.1016/j.ijhydene.2014.01.151 [20] 莫文龙, 马凤云, 刘月娥, 刘景梅, 钟梅, 艾沙·努拉洪.制备方法对Ni-Al2 O3催化剂在CO2-CH4重整反应中催化性能的影响[J].燃料化学学报, 2015, 43(9):1083-1091. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18693.shtmlMO Wen-long, MA Feng-yun, LIU Yue-e, LIU Jing-mei, ZHONG Mei, AISHA·Nulahong. Effect of preparation methods on the catalytic performance of Ni-Al2O3 for CO2-CH4 reforming[J]. J Fuel Chem Technol, 2015, 43(9):1083-1091. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract18693.shtml [21] 索掌怀, 徐秀峰, 马华宪, 安立敦.制备方法对Ni/MgO/Al2O3在甲烷与二氧化碳重整反应中催化性能的影响[J].催化学报, 2000, 21(5):411-414. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=chua200005007&dbname=CJFD&dbcode=CJFQSUO Zhang-huai, XU Xiu-feng, MA Hua-xian, AN Li-dun. Influence of preparation methods on catalytic performance of Ni/MgO/Al2O3 in CO2 reforming of CH4 [J]. Chin J Catal, 2000, 21(5):411-414. http://kns.cnki.net/KCMS/detail/detail.aspx?filename=chua200005007&dbname=CJFD&dbcode=CJFQ [22] 徐军科, 任克威, 周伟, 王晓蕾, 李兆静, 潘相敏, 马建新.制备方法对甲烷干重整催化剂Ni/La2O3/Al2O3结构及性能的影响[J].燃料化学学报, 2009, 37(4):473-479. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract17477.shtmlXU Jun-ke, REN Ke-wei, ZHOU Wei, WANG Xiao-lei, LI Zhao-jing, PAN Xiang-min, MA Jian-xin. Influence of preparation method on the properties and catalytic performance of Ni/La2O3/Al2O3 catalyst for dry reforming of methane[J]. J Fuel Chem Technol, 2009, 37(4):473-479. http://rlhxxb.sxicc.ac.cn/CN/abstract/abstract17477.shtml [23] CHEN L, ZHU Q, WU R. Effect of Co-Ni ratio on the activity and stability of Co-Mi bimetallic aerogel catalyst for methane oxy-CO2 reforming[J]. Int J Hydrogen Energy, 2011, 36(3):2128-2136. doi: 10.1016/j.ijhydene.2010.11.042 [24] FAN M S, ABDULLAH A Z, BHATIA S. Utilization of greenhouse gases through carbon dioxide reforming of methane over Ni-Co/MgO-ZrO2:Preparation, characterization and activity studies[J]. Appl Catal B:Environ, 2010, 100(1/2):365-377. http://www.sciencedirect.com/science/article/pii/S0926337310003668 [25] HUO J, JING J, LI W. Reduction time effect on structure and performance of ni-co/mgo catalyst for carbon dioxide reforming of methane[J]. Int J Hydrogen Energy, 2014, 39(36):21015-21023. doi: 10.1016/j.ijhydene.2014.10.086 [26] KITTISAKMONTREE P, PONGTHAWORNSAKUN B, YOSHIDA H, FUJITA S I, ARAI M, PANPRANOT J. The liquid-phase hydrogenation of 1-heptyne over Pd-Au/TiO2 catalysts prepared by the combination of incipient wetness impregnation and deposition-precipitation[J]. J Catal, 2013, 297(1):155-164. http://www.sciencedirect.com/science/article/pii/S002195171200320X [27] SANDOVAL A, AGUIAR A, LOUIS C, TRAVERSE A, ZANELLA R. Bimetallic Au-Ag/TiO2 catalyst prepared by deposition-precipitation:High activity and stability in CO oxidation[J]. J Catal, 2011, 281(1):40-49. doi: 10.1016/j.jcat.2011.04.003 [28] PUTLURU S S R, SCHILL L, JENSEN A D, SIRET B, TABARIES F, FEHRMANN R. Mn/TiO2 and Mn-Fe/TiO2 catalysts synthesized by deposition precipitation-promising for selective catalytic reduction of NO with NH3 at low temperature[J]. Appl Catal B:Environ, 2015, 165:628-635. doi: 10.1016/j.apcatb.2014.10.060 [29] SIDIK S M, TRIWAHYONO S, JALILA A, MAJID Z A, SALAMUN N, TALIB N B, ABDULLAH T A T. CO2 reforming of CH4 over Ni-Co/MSN for syngas production:Role of Co as a binder and optimization using rsm[J]. Chem Eng J, 2016, 295:1-10. doi: 10.1016/j.cej.2016.03.041 [30] ROSTRUP-NIELSEN J R. Coking on nickel catalysts for steam reforming of hydrocarbons[J]. J Catal, 1974, 33(2):184-201. doi: 10.1016/0021-9517(74)90263-2 -





 下载:
下载: