Effect of preparation method on the structure and properties of coal tar model compound cracking catalyst Ni/Al2O3
-
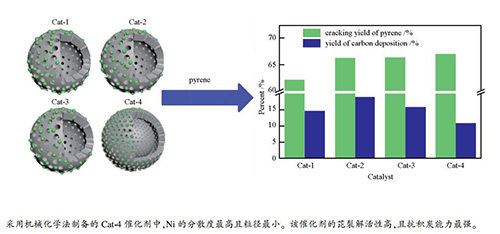
摘要: 采用等体积浸渍法、浸渍沉淀法和机械化学法(市售载体和自制载体)制备了Cat-1、Cat-2、Cat-3和Cat-4四种催化剂,通过BET、H2-TPR、XRD、XPS和NH3-TPD等表征催化剂的结构特征,考察了各催化剂对煤焦油模型化合物甲苯和芘(3%,质量分数)裂解反应性能的影响。结果表明,四种催化剂均为介孔材料,且Cat-4的介孔有序度更高,比表面积最大,达235 m2/g。Cat-4催化剂中,NiAl2O4尖晶石的还原峰面积最高,占总面积的85.2%,还原后Ni的分散度最大,粒径最小,约为10.0 nm,意味着活性位点多。实验表明,除Cat-1外,其他催化剂对芘的裂解活性基本相当,其中,Cat-4作用下的析碳量最低,为10.84%,经Cat-1、Cat-2和Cat-3裂解后,体系中的析碳量分别较Cat-4增加了35.0%、74.7%和45.7%。可见,机械化学法制备的催化剂不仅具有最高的比表面积利于活性组分分散,而且NiAl2O4尖晶石含量最高,可抑制裂解过程中积炭的生成,因而最适宜于甲苯+芘裂解体系。
-
关键词:
- 制备方法 /
- Ni/Al2O3催化剂 /
- 模型化合物 /
- 裂解性能
Abstract: Cat-1, Cat-2, Cat-3 and Cat-4 catalysts were synthesized via different preparation methods that are incipient wetness, impregnation-precipitation and mechanochemical method (carriers from market and homemade for comparison), and then characterized by BET, H2-TPR, XRD, XPS and NH3-TPD. The cracking behavior of toluene and pyrene (3%, mass fraction) (coal tar model compounds) were investigated to evaluate the catalytic performance of the stated catalysts. The catalyst characterization showed that the pore size of all the catalysts belonged to mesoporous range, and Cat-4 catalyst exhibited higher ordered mesoporous and larger surface area than others, up to 235 m2/g. Besides, the peak area of NiAl2O4 spinel reached up to the highest value of 85.2%. The dispersion of Ni in the reduced Cat-4 was the highest and its particle size was the lowest value, about 10.0 nm. Which means that there are more active sites. The catalytic performance results showed that the cracking rate of pyrene varied little for other catalysts, except for Cat-1, but the lowest carbon deposition of 10.84% was obtained under the action of Cat-4, while the carbon deposition of Cat-1, Cat-2 and Cat-3 increased by 35.0%, 74.7% and 45.7% respectively compared with that of Cat-4. Thus, Cat-4 prepared by mechanochemical method is more suitable for the cracking of toluene and pyrene system because of highest BET surface area, which is favorable for the dispersion of active component, and at the same time, the highest content of NiAl2O4 can inhibit the formation of carbon.-
Key words:
- preparation method /
- Ni/Al2O3 catalyst /
- model compound /
- cracking performance
-
图 1 催化剂评价装置示意图
1: N2 cylinder; 2: flowmeter; 3: pump; 4: sample bulb; 5: thermocouple; 6: vaporizer; 7: pyrolyzer; 8: product sorage tank; 9: wet type flowmeter; 10: drying tower; 11: reservoir bag; 12: gas chromatograph; 13: cold hydrazine
Figure 1 Schematic diagram of the catalytic evaluation device
表 1 催化剂的EDX表征
Table 1 EDX results of the catalysts
Catalyst Ni w /% Loading ratio/% experimental value theoretical value Cat-1 14.66 15.00 97.73 Cat-2 14.57 97.13 Cat-3 14.35 95.67 Cat-4 14.32 95.47 表 2 催化剂的孔结构特征
Table 2 Pore characteristics of the catalysts
Catalyst ABET /(m2·g-1) d /nm v /(cm3·g-1) Cat-1 123 11.41 0.32 Cat-2 123 10.78 0.33 Cat-3 107 10.83 0.29 Cat-4 235 8.20 0.48 表 3 不同Ni物种的分散度和还原峰面积比例
Table 3 Dispersion of different Ni species and the proportion of reduction peak area
Catalyst tm /℃ Fraction of total area /% Ni metal area /(m 2·g -1) Dispersion /% Ni metal particle size d/nm β γ β γ Cat-1 702 895 25.7 74.3 1.6 1.6 18.8 Cat-2 700 891 28.3 71.7 4.1 4.2 15.2 Cat-3 776 886 26.5 73.5 4.0 4.2 14.8 Cat-4 737 859 14.8 85.2 4.4 4.6 10.0 表 4 催化剂对模型化合物催化裂解产率的影响
Table 4 Catalytic cracking yield of the model compounds over the catalysts
Catalyst Model compound Liquid yield/% Gasyield/% Yield of carbondeposition/% Cracking yieldof pyrene/% Pyrene production rate/(mg·g -1) Cat-1 toluene 83.83 1.56 14.60 - 0.11 toluene + pyrene 83.81 1.56 14.63 62.14 - Cat-2 toluene 78.35 2.78 18.87 - 0.00 toluene + pyrene 78.27 2.79 18.94 66.30 - Cat-3 toluene 82.11 2.13 15.76 - 0.32 toluene + pyrene 82.06 2.15 15.79 66.41 - Cat-4 toluene 86.67 2.51 10.82 - 0.93 toluene + pyrene 86.51 2.65 10.84 66.98 - 表 5 裂解反应液体产物GC-MS谱图的定性分析
Table 5 Qualitative analysis of GC-MS spectrum of liquid product from the cracking reaction
Peak Name Structure Peak Name Structure 1 toluene 
8 m-methylbiphenyl 
2 p-xylene 9 2, 2′-dimethylbiphenyl 3 o-xylene 10 3, 3′-dimethylbiphenyl 4 m-xylene 11 cis-stilbene 5 naphthalene 12 phenanthrene 6 2-ethenylnaphthalene1 13 pyrene 7 diphenylmethane 表 6 试样表面积炭的Raman特性
Table 6 Raman property of carbon deposition on the catalysts after evaluation experiment
Catalyst Average carbon formation rate /(mgc ·gcat-1·min-1) Relative intensity ratio of D and G bands on Raman apectra (ID/ IG) Cat-1 0.0197 1.237 Cat-2 0.0231 1.098 Cat-3 0.0214 1.199 Cat-4 0.0132 0.970 -
[1] KAN T, WANG H Y, HE H X, LI C S, ZHANG S J. Experimental study on two-stage catalytic hydroprocessing of middle-temperature coal tar to clean liquid fuels[J]. Fuel, 2011, 90(11):3404-3409. doi: 10.1016/j.fuel.2011.06.012 [2] 高晋生.煤的热解、炼焦和煤焦油加工[M].北京:化学工业出版社, 2010.GAO Jin-sheng. The Coal Pyrolysis, Coke and Coal Tar Processing[M]. Beijing:Chemical Industry Press, 2010. [3] 王向辉, 门卓武, 许明, 翁丽, 刘科.低阶煤粉煤热解提质技术研究现状及发展建议[J].洁净煤技术, 2014, 20(6):36-41. http://d.old.wanfangdata.com.cn/Periodical/jjmjs201406010WANG Xiang-hui, MEN Zhuo-wu, XU Ming, WENG Li, LIU Ke. Research status and development proposals on pyrolysis techniques of low rank pulverized coal[J]. Clean Coal Technol, 2014, 20(6):36-41. http://d.old.wanfangdata.com.cn/Periodical/jjmjs201406010 [4] 侯斌, 吕子安, 李晓辉, 李定凯.生物质热解产物中焦油的催化裂解[J].燃料化学学报, 2001, 29(1):70-75. doi: 10.3969/j.issn.0253-2409.2001.01.014HOU Bin, LÜ Zi-an, LI Xiao-hui, LI Ding-kai. Catalytic cracking of tar derived from biomass pyrolysis[J]. J Fuel Chem Technol, 2001, 29(1):70-75. doi: 10.3969/j.issn.0253-2409.2001.01.014 [5] 杨修春, 韦亚南, 李伟捷.焦油裂解用催化剂的研究进展[J].化工进展, 2007, 26(3):326-330. doi: 10.3321/j.issn:1000-6613.2007.03.006YANG Xiu-chun, Wei Ya-nan, LI Wei-jie. Research progress of catalysts for tar cracking[J]. Chem Ind Eng Progress, 2007, 26(3):326-330. doi: 10.3321/j.issn:1000-6613.2007.03.006 [6] 余长林, 胡久彪, 杨凯, 周晓春.制备方法对Ni/CeO2-Al2O3催化剂甲烷部分氧化催化性能的影响[J].燃料化学学报, 2013, 41(6):722-728. doi: 10.3969/j.issn.0253-2409.2013.06.013YU Chang-lin, HU Jiu-biao, YANG Kai, ZHOU Xiao-chun. Effects of preparation methods on the catalytic performance of Ni/CeO2-Al2O3 catalyst in methane partial oxidation[J]. J Fuel Chem Technol, 2013, 41(6):722-728. doi: 10.3969/j.issn.0253-2409.2013.06.013 [7] 徐军科, 任克威, 周伟, 王晓蕾, 李兆静, 潘相敏, 马建新.制备方法对甲烷干重整催化剂Ni/La2O3/Al2O3结构及性能的影响[J].燃料化学学报, 2009, 37(4):473-479. doi: 10.3969/j.issn.0253-2409.2009.04.017XU Jun-ke, REN Ke-wei, ZHOU Wei, WANG Xiao-lei, LI Zhao-jing, PAN Xiang-min, MA Jian-xin. Influence of preparation method on the properties and catalytic performance of Ni/La2O3/Al2O3 catalyst for dry reforming of methane[J]. J Fuel Chem Technol, 2009, 37(4):473-479. doi: 10.3969/j.issn.0253-2409.2009.04.017 [8] 罗来涛, 黄红冈.镍基海泡石催化剂制备方法与表面性质的研究[J].工业催化, 2000, 8(3):60-64. doi: 10.3969/j.issn.1008-1143.2000.03.012LUO Lai-tao, HUANG Hong-gang. Study on preparation and surface behavior of nickel sepiolite catalyst[J]. Ind Catal, 2000, 8(3):60-64. doi: 10.3969/j.issn.1008-1143.2000.03.012 [9] 柴永明, 相春娥, 孔会清, 柳云骐, 刘晨光.馏分油浆态床加氢处理研究Ⅰ催化剂制备方法[J].燃料化学学报, 2008, 36(6):720-725. doi: 10.3969/j.issn.0253-2409.2008.06.014CHAI Yong-ming, XIANG Chun-e, KONG Hui-qing, LIU Yun-qi, LIU Chen-guang. A study on slurry-bed hydrotreating process of distillate oil:ⅠCatalyst preparation[J]. J Fuel Chem Technol, 2008, 36(6):720-725. doi: 10.3969/j.issn.0253-2409.2008.06.014 [10] 王广建, 邴连成, 郭娜娜, 杨志坚, 张健康.浸渍沉淀法制备活性炭负载Co-Mo双金属脱硫催化剂[J].燃料化学学报, 2012, 40(10):1252-1257. doi: 10.3969/j.issn.0253-2409.2012.10.015WANG Guang-jian, BING Lian-cheng, GUO Na-na, YANG Zhi-jian, ZHANG Jian-kang. Preparation of activated carbon-supported Co-Mo bimetallic catalyst by impregnation-precipitation method[J]. J Fuel Chem Technol, 2012, 40(10):1252-1257. doi: 10.3969/j.issn.0253-2409.2012.10.015 [11] AMIN M N, LI Y, RAZZAQ R, LU X M, LI C S, ZHANG S J. Pyrolysis of low rank coal by nickel based zeolite catalysts in the two-staged bed reactor[J]. J Anal Appl Pyrolysis, 2016, 118(MAR):54-62. http://cn.bing.com/academic/profile?id=5d3ec09d9c1ab1a40dd4c9144039e9e1&encoded=0&v=paper_preview&mkt=zh-cn [12] HAN J Z, LIU X X, YUE J R, XI B F, GAO S Q, XU G W. Catalytic upgrading of in situ coal pyrolysis tar over Ni-Char catalyst with different additives[J]. Energy Fuels, 2014, 28(8):4934-4941. doi: 10.1021/ef500927d [13] HU F X, YANG G H, DING G Z, LI Z, DU K S, HU Z F, TIAN S R. Experimental study on catalytic cracking of model tar compounds in a dual layer granular bed filter[J]. Appl Energy, 2016, 170:47-57. doi: 10.1016/j.apenergy.2016.02.080 [14] 邹梦, 马凤云, 莫文龙, 孔令涛, 刘景梅, 钟梅, 肖艳.机械化学法制备甲烷化Ni/Al2O3催化剂性能研究[J].应用化工, 2017, 46(12):2314-2319. doi: 10.3969/j.issn.1671-3206.2017.12.008ZOU Meng, MA Feng-yun, MO Wen-long, KONG Ling-tao, LIU Jing-mei, ZHONG Mei, XIAO Yan. Mechanochemical prepared Ni/Al2O3 catalysts and their catalytic performance for methanation[J]. Appl Chem Ind, 2017, 46(12):2314-2319. doi: 10.3969/j.issn.1671-3206.2017.12.008 [15] 张艳敏, 邹达, 赵渊, 钟梅, 马凤云.双金属催化剂对煤焦油模型化合物催化裂解行为的影响[J].化工学报, 2017, 68(10):3805-3815. http://d.old.wanfangdata.com.cn/Periodical/hgxb201710018ZHANG Yan-min, ZOU Da, ZHAO Yuan, ZHONG Mei, MA Feng-yun. Effect of bimetallic catalysts on cracking behavior of coal tar model compounds[J]. CIESC J, 2017, 68(10):3805-3815. http://d.old.wanfangdata.com.cn/Periodical/hgxb201710018 [16] CAO T T, SONG Z G, WANG S B, CAO X X, LI Y, XIA J. Characterizing the pore structure in the silurian and permian shales of the sichuan basin, China[J]. Mar Petrol Geol, 2015, 61:140-150. doi: 10.1016/j.marpetgeo.2014.12.007 [17] 杨华明, 欧阳静, 张科, 史蓉蓉, 张向超.机械化学合成纳米材料的研究进展[J].化工进展, 2005, 24(3):239-244. doi: 10.3321/j.issn:1000-6613.2005.03.004YANG Hua-ming, OUYANG Jin, ZHANG Ke, SHI Rong-rong, ZHANG Xiang-chao. Research progress of mechano-chemical preparation of nanomaterials[J]. Chem Ind Eng Progress, 2005, 24(3):239-244. doi: 10.3321/j.issn:1000-6613.2005.03.004 [18] 杨圣品, 施雨湘.高能球磨法制备金属微粉的研究[J].焊接技术, 2002, 31(3):43-44. doi: 10.3969/j.issn.1002-025X.2002.03.021YANG Sheng-pin, SHI Yu-xiang. Research of metal powder preparation by high energy ball milling[J]. Welding Technol, 2002, 31(3):43-44. doi: 10.3969/j.issn.1002-025X.2002.03.021 [19] GROEN J C, PEFFER L A A, PEREZ-RAMIREZ J. Pore size determination in modified micro-and mesoporous materials. Pitfalls and limitations in gas adsorption data analysis[J]. Microporous Mesoporous Mater, 2003, 60(1/3):1-17. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=JJ0213448779 [20] SING K S W. Reporting physisorption data for gas/solid systems-with special reference to the determination of surface area and porosity[J]. Pure Appl Chem, 1985, 57(4):603-619. doi: 10.1351/pac198557040603 [21] ZHANG J, XU H Y, JIN X L, GE Q J, LI W Z. Characterizations and activities of the nano-sized Ni/Al2O3 and Ni/La-Al2O3catalysts for NH3 decomposition[J]. Appl Catal A:Gen, 2005, 290(1/2):87-96. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=JJ024426858 [22] BOUKHA Z, JIMENEZ-GONZALEZ C, RIVAS B D, GONZALEZ-VELASCO J R, GUTIERREZ-ORTIZ J I, LOPEZ-FONSECA R. Synthesis, characterisation and performance evaluation of spinel-derived Ni/Al2O3 catalysts for various methane reforming reactions[J]. Appl Catal B:Environ, 2014, 158/159(1):190-201. http://www.wanfangdata.com.cn/details/detail.do?_type=perio&id=JJ0232793414 [23] HU D C, GAO J J, PING Y, JIA L H, GUNAWAN P, ZHONG Z Y, XU G W, GU F N, SU F B. Enhanced investigation of CO methanation over Ni/Al2O3 catalysts for synthetic natural gas production[J]. Ind Eng Chem Res, 2012, 51(13):4875-4886. doi: 10.1021/ie300049f [24] SUN N N, WEN X, WANG F, PENG W C, XIAO F K, WEI W, SUN Y H. Influence of Ni content on catalytic performance of Ni-CaOZrO2 catalysts in CH4-CO2 reforming[J]. Fine Chem, 2010, 27(10):1004-1008. [25] 莫文龙, 马凤云, 刘月娥, 刘景梅, 钟梅, 艾沙·努拉洪.溶液燃烧法制备Ni-Al2O3催化剂用于CO2-CH4重整研究[J].无机材料学报, 2016, 31(5):485-491. http://d.old.wanfangdata.com.cn/Periodical/wjclxb201605007MO Wen-long, MA Feng-yun, LIU Yue-e, LIU Jing-mei, ZHONG Mei, AISHA·nulahong. Preparation of Ni-Al2O3 catalysts by solution combustion method for CO2 reforming of CH4[J]. J Inorg Mater, 2016, 31(5):485-491. http://d.old.wanfangdata.com.cn/Periodical/wjclxb201605007 [26] ZHANG J F, BAI Y X, ZHANG Q D, WANG X X, ZHANG T, TIAN Y S, HAN Y Z. Low-temperature methanation of syngas in slurry phase over Zr-doped Ni/γ-Al2O3 catalysts prepared using different methods[J]. Fuel, 2014, 132(15):211-218. [27] CZEKAJ I, LOVIAT F, RAIMONDI F, WAMBACH J, BIOLLAZ S, WOKAUN A. Characterization of surface processes at the Ni-based catalyst during the methanation of biomass-derived synthesis gas:X-ray photoelectron spectroscopy (XPS)[J]. Appl Catal A:Gen, 2007, 329(10):68-78. http://cn.bing.com/academic/profile?id=c5edfa5a6841bfa104c17fdc4073d836&encoded=0&v=paper_preview&mkt=zh-cn [28] SALLEH N F M, JALIL A A, TRIWAHYONO S, EFENDI J, MUKTI R R, HAMEED B H. New insight into electrochemical-induced synthesis of NiAl2O4/Al2O3:Synergistic effect of surface hydroxyl groups and magnetism for enhanced adsorptivity of Pd(Ⅱ)[J]. Appl Surf Sci, 2015, 349(15):485-495. [29] HERACLEOUS E, LEE A F, WILSON K, LEMONIDOU A A. Investigation of Ni-based alumina-supported catalysts for the oxidative dehydrogenation of ethane to ethylene:Structural characterization and reactivity studies[J]. J Catal, 2005, 231(1):159-171. doi: 10.1016/j.jcat.2005.01.015 [30] 宫立倩, 陈吉祥, 邱业君, 张继炎.焙烧温度对甲烷催化部分氧化Ni/MgO-Al2O3催化剂结构和性能的影响[J].燃料化学学报, 2005, 33(2):224-228. doi: 10.3969/j.issn.0253-2409.2005.02.019GONG Li-qian CHEN Ji-xiang QIU Ye-jun ZHANG Ji-yan. Effects of calcinations temperature on structure and catalytic performance of Ni/MgO-Al2O3 catalysts for partial oxidation of methane[J]. J Fuel Chem Technol, 2005, 33(2):224-228. doi: 10.3969/j.issn.0253-2409.2005.02.019 [31] CHEN Y G, REN J. Conversion of methane and carbon dioxide into synthesis gas over alumina-supported nickel catalysts. Effect of Ni-Al2O3, interactions[J]. Catal Lett, 1994, 29(1/2):39-48. [32] BHATTACHARYYA A, CHANG V W. CO2 reforming of methane to syngas:Deactivation behavior of nickel aluminate spinel catalysts[J]. Stud Surface Sci Catal, 1994, 88:207-213. doi: 10.1016/S0167-2991(08)62742-1 [33] MO W L, MA F Y, LIU Y E, LIU J M, ZHONG M, AISHA·N L H. Preparation of porous Al2O3 by template method and its application in Ni-based catalyst for CH4/CO2 reforming to produce syngas[J]. Int J Hydrogen Energy, 2015, 40(46):16147-16158. doi: 10.1016/j.ijhydene.2015.09.149 [34] GUO J J, LOU H, ZHENG X M. The deposition of coke from methane on a Ni/MgAl2O4 catalyst[J]. Carbon, 2007, 45(6):1314-1321. doi: 10.1016/j.carbon.2007.01.011 [35] XU J K, ZHOU W, WANG J H, LI Z J, MA J X. Characterization and analysis of carbon deposited during the dry reforming of methane over Ni/La2O3/Al2O3 Catalysts[J]. Chin J Catal, 2009, 30(11):1076-1084. doi: 10.1016/S1872-2067(08)60139-4 [36] FRUSTERI F, SPADARO L, ARENA F, CHUVILIN A. TEM evidence for factors affecting the genesis of carbon species on bare and K-promoted Ni/MgO catalysts during the dry reforming of methane[J]. Carbon, 2002, 40(7):1063-1070. doi: 10.1016/S0008-6223(01)00243-3 [37] 张兆斌, 余长春, 沈师孔.甲烷部分氧化制合成气的La2O3助Ni/MgAl2O4催化剂[J].催化学报, 2000, 21(1):14-18. doi: 10.3321/j.issn:0253-9837.2000.01.006ZHANG Zhao-bin, YU Chang-chun, SHEN Shi-kong. Partial oxidation of CH4 to syngas on La2O3-promoted Ni/MgAl2O4[J]. Chin J Catal, 2000, 21(1):14-18. doi: 10.3321/j.issn:0253-9837.2000.01.006 [38] 杨帆, 周志杰, 王辅臣, 刘海峰, 龚欣, 于遵宏.氢气存在下的煤焦水蒸气气化:Ⅰ反应特性研究[J].燃料化学学报, 2009, 37(1):36-41. doi: 10.3969/j.issn.0253-2409.2009.01.007YANG Fan, ZHOU Zhi-jie, WANG Fu-chen, LIU Hai-feng, GONG Xin, YU Zun-hong. Coal char gasification with steam and H2:I The gasification reaction characteristics[J]. J Fuel Chem Technol, 2009, 37(1):36-41. doi: 10.3969/j.issn.0253-2409.2009.01.007 -





 下载:
下载: